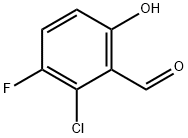
2-chloro-3-fluoro-6-hydroxybenzaldehyde synthesis
- Product Name:2-chloro-3-fluoro-6-hydroxybenzaldehyde
- CAS Number:1263378-00-2
- Molecular formula:C7H4ClFO2
- Molecular Weight:174.56
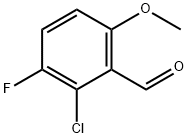
1263378-40-0
19 suppliers
inquiry

1263378-00-2
15 suppliers
inquiry
Yield:1263378-00-2 80%
Reaction Conditions:
Stage #1: 2-chloro-3-fluoro-6-methoxy-benzaldehydewith boron tribromide in dichloromethane at -78 - 20; for 17 h;Inert atmosphere;
Stage #2: with methanol in dichloromethane at 0 - 20; for 0.833333 h;
Steps:
2-Chloro-3-fluoro-6-hydroxybenzaldehyde 2-Chloro-3-fluoro-6-methoxybenzaldehyde (46.0 g, 245 mmol) was added in a three neck flask equipped with a nitrogen inlet, a thermometer and an addition funnel. DCM (800 mL) was added and cooled to -70 to -78° C. using an acetone/dry ice bath. Boron tribromide (25.4 mL, 269 mmol) was diluted in 200 mL of dichloromethane and added to the reaction mixture slowly over a period of 1 h. The reaction mixture was allowed to warm to room temperature and stirred for 16 h. Then the reaction mixture was cooled to 0° C. in an ice bath and quenched by adding methanol (150 mL) over a period of 30 minutes and stirred at room temperature for 20 min. The solvents were removed, and the residue was diluted with dichloromethane and washed with aq. sodium bicarbonate solution followed by water. The organic layer was dried over sodium sulfate, filtered and concentrated to give crude product. It was purified by column chromatography on silica gel eluting with 2→3% methanol in dichloromethane, giving 34 g (80% yield) of the title compound. 1HNMR (300 MHz, CDCl3): δ=11.68 (s, 1H), 10.39 (s, 1H), 7.26-7.35 (m, 1H), 6.86-6.90 (m, 1H).
References:
US2011/281888,2011,A1 Location in patent:Page/Page column 25
261762-39-4
139 suppliers
$8.00/250mg

1263378-00-2
15 suppliers
inquiry