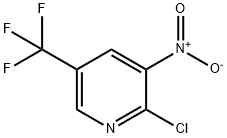
2-CHLORO-3-NITRO-5-(TRIFLUOROMETHYL)PYRIDINE synthesis
- Product Name:2-CHLORO-3-NITRO-5-(TRIFLUOROMETHYL)PYRIDINE
- CAS Number:72587-15-6
- Molecular formula:C6H2ClF3N2O2
- Molecular Weight:226.54
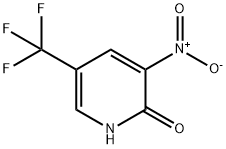
33252-64-1

72587-15-6
General steps for the synthesis of 5-(trifluoromethyl)-3-nitro-2-chloropyridine from 2-hydroxy-3-nitro-5-trifluoromethylpyridine: (2) 52 g of 2-hydroxy-3-nitro-5-trifluoromethylpyridine was added to 150 mL of phosphorochloridic acid chloride, and 21 g of quinoline was slowly added under stirring, and the rate of addition was controlled so that the internal temperature of the reaction system did not exceed 50 °C, while the 2-hydroxy-3-nitro-5-trifluoromethylpyridine gradually dissolved. 3-nitro-5-trifluoromethylpyridine was gradually dissolved. Under the protection of argon gas, the reaction system was heated by an oil bath and the external temperature was set to 120°C. After the reaction system started to reflux for 20-30 min, the system was kept micro-boiling for 1.5 h. The reaction process was monitored by TLC. The progress of the reaction was monitored by TLC to show that the feedstock was fully reacted and then the heating was stopped and the trichlorophosphorus was concentrated under reduced pressure to remove the trichlorophosphorus. The residue was dissolved in 400 mL of ethyl acetate and washed with 2N HCl (200 mL x 2) to remove quinoline. The mixture was subsequently washed with saturated sodium bicarbonate solution to neutral and the organic phase was dried to give 5-(trifluoromethyl)-3-nitro-2-chloropyridine.TLC conditions: raw material Rf = 0.1, product Rf = 0.7, and the ratio of unfolding agent was petroleum ether/ethyl acetate = 15/1. 50 g of a brown oily product was finally obtained in 88% yield.
56-93-9
502 suppliers
$5.00/25g

33252-64-1
168 suppliers
$10.00/250mg

72587-15-6
172 suppliers
$29.71/1g
Yield:72587-15-6 92%
Reaction Conditions:
with trichlorophosphate in acetonitrile; for 3 h;Heating;
Steps:
22.a
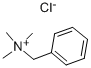
a) Synthesis of 2-chloro-3-nitro-5-trifluoromethyl-pyridine In a 25 ml flask, 3-nitro-5-trifluoromethyl-pyridin-2-ol (300 mg, 1.44 mmol) was dissolved in acetonitrile (4.5 ml), and then phosphorus oxychloride (0.4 ml, 4.33 mmol) and benzyltrimethyl ammonium chloride (164 mg, 0.721 mmol) were added thereto. The mixture was stirred for 3 hours at 800. After completion of the reaction, water was added thereto and the mixture was extracted with ethyl acetate. The combined organic layer was dried over anhydrous sodium sulfate (Na2SO4), filtered and evaporated under reduced pressure to obtain white solid (300 mg, 92%). 1H-NMR (DMSO-d6, 300 MHz); δ=9.21 (s, 1H), 9.08 (s, 1H).
References:
US2011/28467,2011,A1 Location in patent:Page/Page column 22

33252-64-1
168 suppliers
$10.00/250mg

72587-15-6
172 suppliers
$29.71/1g
874483-75-7
0 suppliers
inquiry

33252-64-1
168 suppliers
$10.00/250mg

72587-15-6
172 suppliers
$29.71/1g

33252-64-1
168 suppliers
$10.00/250mg

72587-15-6
172 suppliers
$29.71/1g
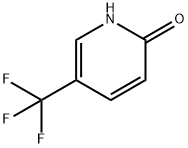
33252-63-0
295 suppliers
$10.00/1g

72587-15-6
172 suppliers
$29.71/1g