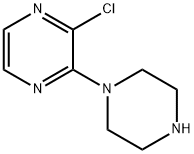
2-CHLORO-3-(PIPERAZINYL)PYRAZINE synthesis
- Product Name:2-CHLORO-3-(PIPERAZINYL)PYRAZINE
- CAS Number:85386-99-8
- Molecular formula:C8H11ClN4
- Molecular Weight:198.65
![3'-CHLORO-2,3,5,6-TETRAHYDRO-[1,2']BIPYRAZINYL-4-CARBOXYLIC ACID TERT-BUTYL ESTER](/CAS/GIF/313654-83-0.gif)
313654-83-0

85386-99-8
The general procedure for the synthesis of 2-chloro-3-piperazinopyrazine from tert-butyl 4-(3-chloropyrazin-2-yl)piperazine-1-carboxylate was as follows: tert-butyl 3'-chloro-2,3,5,6-tetrahydro-[1,2']bipyrazinyl-4-carboxylate (10 g, 33.4 mmol, 1 eq.) was dissolved in 1,4-dioxane (160 mL), and 4 M hydrochloric acid in 1,4- dioxane solution (80 mL, 0.3 mol, 10 eq.). The reaction mixture was stirred overnight at room temperature under nitrogen protection. Upon completion of the reaction, the reaction solution was diluted with dichloromethane (600 mL) and subsequently alkalized with 50% aqueous sodium hydroxide. The organic and aqueous layers were separated after addition of water (100 mL). The aqueous layer was extracted twice with dichloromethane (200 mL). All organic extracts were combined, washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, filtered and concentrated to give 3'-chloro-3,4,5,6-tetrahydro-2H-[1,2']bis(pyrazinyl) product as a viscous oil, which solidified on standing (6.39 g, 96% yield). Mass spectrum (electrospray ionization): m/z = 199.1, 201.1 [M + H]+.
![3'-CHLORO-2,3,5,6-TETRAHYDRO-[1,2']BIPYRAZINYL-4-CARBOXYLIC ACID TERT-BUTYL ESTER](/CAS/GIF/313654-83-0.gif)
313654-83-0
48 suppliers
$55.00/50mg

85386-99-8
55 suppliers
$50.00/250mg
Yield: 96%
Reaction Conditions:
Stage #1:2-Chloro-3-(4-tert-butoxycarbonyl-1-piperazinyl)pyrazine with hydrogenchloride in 1,4-dioxane at 20;
Stage #2: with sodium hydroxide in 1,4-dioxane;dichloromethane;water
Steps:
2
To a solution of 3'-chloro-2,3,5,6-tetrahydro-[l,2']bipyrazinyl-4-carboxylic acid t-butyl ester (10 g, 33.4 mmol, 1 eq) in 1,4-dioxane (160 mL) add a 4 M solution of hydrochloric acid in 1,4-dioxane (80 mL, 0.3 mol, 10 eq) and stir under nitrogen at room temperature overnight. Dilute with DCM (600 mL) then basify with 50% aqueous sodium hydroxide. Add water (100 mL), separate the layers and extract the aqueous twice with DCM (200 mL). Combine the organic extracts, wash with saturated aqueous sodium chloride, dry (magnesium sulfate), filter, and concentrate to give 3'-chloro- 3,4,5, 6-tetrahydro-2H-[l,2']bipyrazinyl as a viscous oil which solidifies on standing (6.39 g, 96%). MS (ES): m/z = 199.1, 201.1 [M+H]+.
References:
ELI LILLY AND COMPANY WO2009/29439, 2009, A1 Location in patent:Page/Page column 14