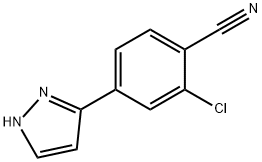
2-Chloro-4-(1H-Pyrazol-5-Yl)Benzonitrile synthesis
- Product Name:2-Chloro-4-(1H-Pyrazol-5-Yl)Benzonitrile
- CAS Number:1297537-37-1
- Molecular formula:C10H6ClN3
- Molecular Weight:203.63
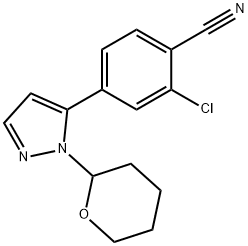
1297537-35-9
16 suppliers
inquiry

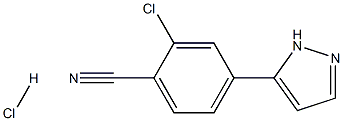
1297537-37-1
132 suppliers
inquiry
Yield:1297537-37-1 95.8%
Reaction Conditions:
Stage #1:2-Chloro-4-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)benzonitrile with hydrogenchloride in water at 10; for 2 h;
Stage #2: with ammonium hydroxide;water in water at 0 - 5; for 4 h;
Steps:
3 Preparation of 2-chloro-4-( 1 H-pyrazol-3 -yl)benzonitrile (V)
2-Chloro-4-( 1 -(tetrahydro-2H-pyran-2y1)- 1 H-pyrazol-5 -yl)benzonitrile (III)(10.0 g, 1.00 eqv.) and methanol (40 ml) were charged. 30 % HC1 (0.3 nil, 0.08 eqv.) was added at 10 ± 3 °C. The mixture was stirred for 2 h at 10 ± 3 °C. Ammonia water (25 %) was added (3.0 nil, 1.1 eqv.) at 10 ± 5 °C. Water (15 nil) was added gradually at 10-20 °C. The mixture was stirred overnight at 20 ± 5 °C. The mixture was then cooled to 0-5 °C and stirred for 4 h at 0-5 °C. The crystalline product was filtered andwashed with cold water:methanol mixture 3:1(30 ml) and dried at 5 0-60 °C. Yield6.78 g (95.8 %). HPLC-purity 99.7 %.
References:
ORION CORPORATION;LAITINEN, Ilpo;KARJALAINEN, Oskari WO2016/162604, 2016, A1 Location in patent:Page/Page column 15
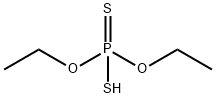
1297537-39-3
11 suppliers
inquiry

1297537-37-1
132 suppliers
inquiry

1297537-39-3
11 suppliers
inquiry
298-06-6
169 suppliers
$62.50/5 g

1297537-37-1
132 suppliers
inquiry
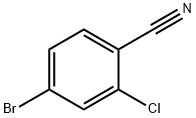
154607-01-9
279 suppliers
$6.00/1g

1297537-37-1
132 suppliers
inquiry
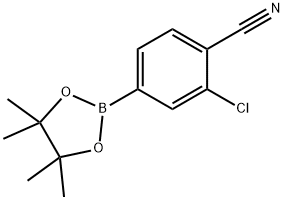
548797-51-9
45 suppliers
$45.00/100mg

1297537-37-1
132 suppliers
inquiry