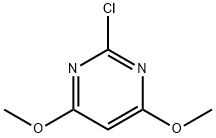
2-Chloro-4,6-dimethoxypyrimidine synthesis
- Product Name:2-Chloro-4,6-dimethoxypyrimidine
- CAS Number:13223-25-1
- Molecular formula:C6H7ClN2O2
- Molecular Weight:174.59
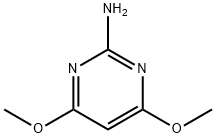
36315-01-2

13223-25-1
General procedure for the synthesis of 2-chloro-4,6-dimethoxypyrimidine from 2-amino-4,6-dimethoxypyrimidine: 1. 1,260 ml of 36% hydrochloric acid was added to a 5-liter four-necked flask and subsequently cooled to 0°C. 2. 180 g (1.16 moles) of 2-amino-4,6-dimethoxypyrimidine was added to the flask in batches and stirred continuously for about 1 hour until the reaction mixture took on a syrupy consistency. 3. After the reaction mixture was further cooled to -15°C, 260 ml of an aqueous solution containing 159 g (2.3 moles) of NaNO2 was slowly added dropwise over a period of about 1 hour under vigorous stirring. 4. after completion of the dropwise addition, the mixture was continued to be stirred at -15°C to -10°C for 1 hour to ensure complete reaction. 5. While keeping the reaction mixture at -5°C, 1.5 liters of 30% aqueous NaOH was slowly added dropwise to adjust the pH to 7 for neutralization. 6. The resulting purple clay-like substance was collected by filtration under reduced pressure. 7. the target compound 2-chloro-4,6-dimethoxypyrimidine was extracted from the clay-like material using 3 liters of ethyl acetate. 8. The organic layer was washed with water, dried over anhydrous sodium sulfate, and the solvent was removed to yield 63 g of light blue crude crystals. 9. Purification and crystallization by silica gel chromatography resulted in 60.8 g of white crystals (yield: 29.9%) with a melting point of 101.5°-102.5°C. The final product was purified by silica gel chromatography.

36315-01-2
523 suppliers
$18.60/2.5g

13223-25-1
275 suppliers
$6.00/1g
Yield:13223-25-1 29.9%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;sodium nitrite in water;ethyl acetate
Steps:
R.1 Preparation of 2-chloro-4,6-dimethoxypyrimidine
REFERENTIAL EXAMPLE 1 Preparation of 2-chloro-4,6-dimethoxypyrimidine 1,260 ml of 36% hydrochloric acid were charged in a 5-l four-necked flask and then cooled to 0° C. After 180 g (1.16 moles) of 2-amino-4,6-dimethoxypyrimidine were added in small portions into the flask, the resulting mixture was stirred for about 1 hour until the reaction mixture changed into a syrupy form. After the reaction mixture was cooled to -15° C., 260 ml of 159 g (2.3 moles) of NaNO2 in H2 O were added dropwise over about 1 hour under vigorous stirring. After completion of the dropwise addition, the resulting mixture was stirred at -15° to -10° C. for additional 1 hour so that the reaction was brought to completion. While the reaction mixture was retained at -5° C., 1.5 l of a 30% aqueous solution of NaOH were charged dropwise so that the reaction mixture was neutralized to pH 7. By filtration under reduced pressure, a clay-like material of a purple color was collected. The target compound was extracted from the clay-like material using 3 l of ethyl acetate. Through the procedures of washing with water, drying over anhydrous sodium sulfate and removal of the solvent, 63 g of bluish crude crystals were obtained. They were crystallized further by silica gel chromatography to obtain 60.8 g of white crystals (yield: 29.9%). Melting point: 101.5°-102.5° C.
References:
Mitsui Toatsu Chemicals, Incorporated US4986846, 1991, A