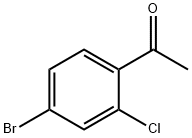
2-Chloro-4-bromoacetophenone synthesis
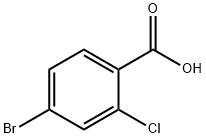
- Product Name:2-Chloro-4-bromoacetophenone
- CAS Number:252561-81-2
- Molecular formula:C8H6BrClO
- Molecular Weight:233.49
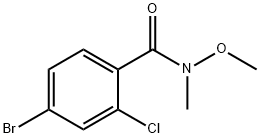
875306-64-2

252561-81-2
Step 6-J: Methylmagnesium bromide (44 mL, 61.6 mmol, 1.4 M in tetrahydrofuran) was slowly added dropwise to tetrahydrofuran (50 mL) at -78 °C. Subsequently, a solution consisting of 4-bromo-2-chloro-N-methoxy-N-methylbenzamide (3.52 g, 12.7 mmol, from Step 6-I) dissolved in tetrahydrofuran (30 mL) was added. The reaction mixture was stirred at -78°C for 45 minutes, then brought to room temperature and continued stirring for 1.5 hours. After completion of the reaction, the mixture was extracted with ethyl acetate (3 times), the organic phases were combined, washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. The crude product was purified by recrystallization (solvent: ethyl acetate/hexane) to afford 1-(4-bromo-2-chlorophenyl)ethanone as a white solid (1.1 g, 37% yield).

75-16-1
274 suppliers
$12.00/10ml

875306-64-2
4 suppliers
inquiry

252561-81-2
119 suppliers
$10.00/250mg
Yield: 86%
Reaction Conditions:
in tetrahydrofuran at 8; for 6 h;
Steps:
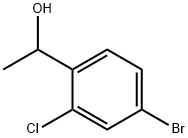
21.2 2. Synthesis of 1-(4-bromo-2-chlorophenyl)ethan-1-one
To a solution of 4-bromo-2-chloro-N-methoxy-N-methylbenzamide (5.6 g, 20 mmol, 1 eq.) in THF (80 mL) was added MeMgBr (3 M, 10 mL, 30 mmol, 1.5 eq.) at 8 °C. The reaction mixture was stirred at 8 °C for 6 h and then was concentrated in vacuo. The residue was dissolved in EtOAc (100 mL) and was poured into saturated aqueous NH4Cl solution (200 mL). The layers were separated and the aqueous layer was extracted with EtOAc (100 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated in vacuo to give 1-(4- bromo-2-chlorophenyl)ethan-1-one as a light brown solid (4 g, yield: 86%), which was carried forward without further purification. 1H NMR: (400 MHz, CDCl3) d: 7.60 (s, 1H), 7.48-7.43 (m, 2H), 2.63 (s, 3H)
References:
BIOGEN MA INC.;HOPKINS, Brian, T.;MA, Bin;PRINCE, Robin;MARX, Isaac;SCHULZ, Jürgen;NEVALAINEN, Marta WO2020/232330, 2020, A1 Location in patent:Page/Page column 98; 99

875306-64-2
4 suppliers
inquiry

252561-81-2
119 suppliers
$10.00/250mg
625446-26-6
0 suppliers
inquiry

75-16-1
274 suppliers
$12.00/10ml

252561-81-2
119 suppliers
$10.00/250mg
1002309-96-7
4 suppliers
inquiry

252561-81-2
119 suppliers
$10.00/250mg
59748-90-2
299 suppliers
$8.00/1g

252561-81-2
119 suppliers
$10.00/250mg