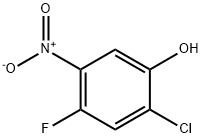
2-Chloro-4-fluoro-5-nitrophenol synthesis
- Product Name:2-Chloro-4-fluoro-5-nitrophenol
- CAS Number:84478-75-1
- Molecular formula:C6H3ClFNO3
- Molecular Weight:191.54
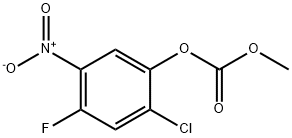
84478-89-7

84478-75-1
2-Chloro-4-fluoro-5-nitrophenyl methyl carbonate (120 g, 0.48 mol) was mixed with sodium hydroxide (22.7 g, 0.57 mol) in water (300 mL) and the reaction was carried out at reflux for 4 hrs. Upon completion of the reaction, the insoluble solids were removed by filtration and the filtrate was acidified with dilute hydrochloric acid to pH < 7. Subsequently, the precipitated solid product was collected by filtration, washed with water and dried to afford 2-chloro-4-fluoro-5-nitrophenol (90 g, 98% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 11.18 (s, 1H), 8.10 (d, J=10.4 Hz, 1H), 7.62 (d, J=7.2 Hz, 1H); mass spectrum (ESI) m/z: 192.1 ([M+H]+).
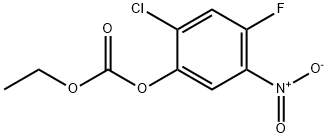
153471-75-1
23 suppliers
$490.00/1g

84478-75-1
196 suppliers
$10.00/1g
Yield:84478-75-1 98%
Reaction Conditions:
with water;sodium hydrogencarbonate in methanol at 20; for 16 h;
Steps:
47.47C
To a solution of the product from Example 47B (0.87 g, 3.30 mmol) in methanol (20 mL) and water (1 mL) was added sodium bicarbonate (2.22 g, 26.4 mmol) and the mixture stirred at room temperature for 16 hours. The methanol was then removed under vacuum, dichloromethane (20 mL) was added to the mixture, the organic solution was washed with brine (50 mL), dried over anhydrous magnesium sulfate, filtered, and concentrated under vacuum to provide the title product (0.62 g, 98%).
References:
ABBOTT LABORATORIES WO2008/133753, 2008, A2 Location in patent:Page/Page column 88

84478-89-7
15 suppliers
inquiry

84478-75-1
196 suppliers
$10.00/1g
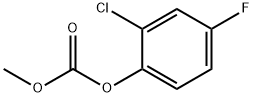
84478-88-6
6 suppliers
$108.00/1g

84478-75-1
196 suppliers
$10.00/1g
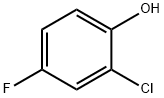
1996-41-4
242 suppliers
$7.00/5g

84478-75-1
196 suppliers
$10.00/1g
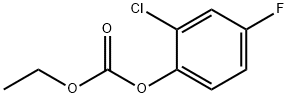
153406-19-0
6 suppliers
inquiry

84478-75-1
196 suppliers
$10.00/1g