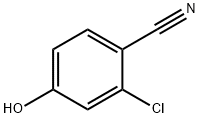
2-CHLORO-4-HYDROXYBENZONITRILE synthesis
- Product Name:2-CHLORO-4-HYDROXYBENZONITRILE
- CAS Number:3336-16-1
- Molecular formula:C7H4ClNO
- Molecular Weight:153.57
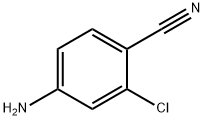
20925-27-3

3336-16-1
Step 5: 2-Chloro-4-aminobenzonitrile (25 g, 0.187 mol) was added to a stirred mixture of concentrated sulfuric acid (58 g) and water (570 mL) and heated between 49 °C and 61 °C for about 30 minutes. The suspension was cooled to about 0°C and a cold solution of sodium nitrite (13.2 g, 0.187 mol) dissolved in water (125 mL) was slowly added dropwise over a temperature range of 0°C to 6°C. After the dropwise addition was completed, stirring of the reaction mixture was continued for about 3 hours. Subsequently, urea (1.5 g) was added to the mixture to quench the excess sodium nitrite. Insoluble impurities were removed by filtration. To the filtrate was added a 50% aqueous sulfuric acid solution (600 mL) while the reaction mixture was heated to between 74 °C and 81 °C with continuous stirring until the nitrogen stopped escaping. Upon completion of the reaction, the product was separated by filtration and purified with recrystallized water to give 2-chloro-4-hydroxybenzonitrile (17.9 g, 62.5% yield, melting point 160 °C).

20925-27-3
176 suppliers
$10.00/5g

3336-16-1
115 suppliers
$13.00/250mg
Yield: 62.5%
Reaction Conditions:
with urea;sodium nitrite in sulfuric acid;water
Steps:
1.5 Step 5
Step 5 A mixture of 2-chloro-4-aminobenzonitrile (25 g, 0.187 m) in stirred concentrated sulfuric acid (58 g) and water (570 ml) was heated at between 49° and 61° C. for approximately thirty minutes. The suspension was cooled to approximately 0° C. and a cold solution of sodium nitrite (13.2 g, 0.187 m) in water (125 ml) was added dropwise at a solution temperature of between 0° and 6° C. Stirring continued thereafter for approximately three hours and then urea (1.5 g) was added to the mixture. The resulting insoluble impurity was filtered off. An aqueous solution of 50% sulfuric acid (600 ml) was added while the reaction mixture was heated at a temperature between 74° and 81° C. and stirred until the evolution of nitrogen gas ceased. The reaction product was separated by filtration and then recrystallized from water to yield 2-chloro-4-hydroxybenzonitrile (17.9 g, 62.5%, mp 160°
References:
Timex Corporation US4424371, 1984, A