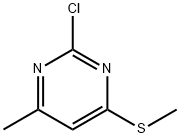
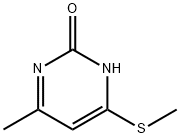
2-CHLORO-4-METHYL-6-METHYLSULFANYL-PYRIMIDINE synthesis
- Product Name:2-CHLORO-4-METHYL-6-METHYLSULFANYL-PYRIMIDINE
- CAS Number:89466-59-1
- Molecular formula:C6H7ClN2S
- Molecular Weight:174.65
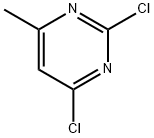
5424-21-5
5188-07-8

89466-59-1
Step 1: Synthesis of 2-chloro-4-methyl-6-(methylthio)pyrimidine. 2,4-Dichloro-6-methylpyrimidine (20.0 g, 0.123 mol) was dissolved in tetrahydrofuran (THF, 200 mL). Sodium methanethiol (20% aqueous solution, 43 g, 0.129 mol) was added slowly and dropwise at -5 °C. After the dropwise addition was completed, the reaction mixture was stirred at room temperature overnight. After completion of the reaction, water (100 mL) and ethyl acetate (EtOAc, 100 mL) were added for extraction and the organic layer was separated. The organic layer was washed twice with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give a yellow solid. The solid was washed with petroleum ether (PE, 100 mL) to afford the target product 2-chloro-4-methyl-6-(methylthio)pyrimidine (9.1 g). Mass spectrometry (electrospray positive ion mode, ES+) analysis: the theoretical molecular weight of C6H7ClN2S was 174 and the measured value was 175 [M+H]+.

5424-21-5
325 suppliers
$10.00/5g
5188-07-8
298 suppliers
$13.00/5g

89466-59-1
88 suppliers
$26.00/250mg
Yield:89466-59-1 61%
Reaction Conditions:
in tetrahydrofuran;water at -10; for 2 h;Inert atmosphere;
Steps:
12 Synthesis of 2-chloro-4-methyl-6-(methylthio)pyrimidine
To a stirred solution of 2. 4-dichloro--6-methylpyrimidine 1 (200 tug, 1 22 mmol) under argon atmosphere in TNT (10 mL.) was added sodium methanethiolate (103 mg, 1.47 rumol in 4 mL of water) at -10 °C and stirred for 2 h. The reaction was monitored by TLC; after completion of the reaction, the reaction mixture was diluted with water (20 mL) and extracted. with EtO.Ac (2 x 30 niL). The combined organic extracts were dried over sod jum sulfate. filtered and concentrated in vacio to obtain the crude prodtwt. The crude product was purified through silica gel column chromatography using 3% EtOAc hexanes to afford compound 166 (1 30 mg, 61%) as a white solid. TLC: 5% EtOAc/ Toluene (R,’: 08) ‘H-NMR (DMSO-d6, 400 MHz): 67.38 (s, 11-1;. 2.53 (s. 311). 2.37 (5, 311).
References:
WO2015/57945,2015,A1 Location in patent:Paragraph 00127
16710-11-5
45 suppliers
$28.60/50mg

89466-59-1
88 suppliers
$26.00/250mg