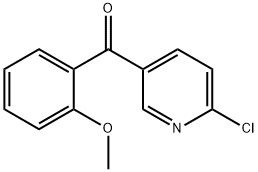
2-Chloro-5-(2-methoxybenzoyl)pyridine synthesis
- Product Name:2-Chloro-5-(2-methoxybenzoyl)pyridine
- CAS Number:86580-59-8
- Molecular formula:C13H10ClNO2
- Molecular Weight:247.68
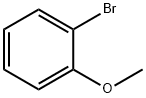
578-57-4
307 suppliers
$6.00/10g
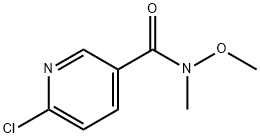
149281-42-5
50 suppliers
$60.00/100mg

86580-59-8
14 suppliers
$160.00/100mg
Yield:86580-59-8 47.6%
Reaction Conditions:
Stage #1: 2-bromoanisolewith n-butyllithium in tetrahydrofuran;hexane at -78;Inert atmosphere;
Stage #2: 6-chloro-N-methoxy-N-methylpyridine-3-carboxamide in tetrahydrofuran;hexane at -78 - 20;
Steps:
b
To a solution of 1-bromo-2-methoxy-benzene (49.2 g, 0.263 mol) in dry THF (500 mL) was added dropwise n-BuLi (2.5 M in hexane, 105 mL, 0.263 mol) at -78° C. under N2 atmosphere. The mixture was stirred at this temperature for 1 hr and then a solution of 6-chloro-N-methoxy-N-methyl-nicotinamide (50 g, 0.25 mol) in THF (100 mL) was added dropwise. The reaction mixture was warmed to room temperature, stirred overnight and then was quenched with a saturated solution of NH4Cl (300 mL). The separated aqueous layer was extracted with ethyl acetate (3*300 mL). The combined organic layers were washed with brine, dried over Na2SO4, evaporated in vacuo to give the crude product that was purified by column chromatography to yield (6-chloropyridin-3-yl)-(2-methoxyphenyl)-methanone as a white solid (31 g, 47.6%).
References:
US2009/246137,2009,A1 Location in patent:Page/Page column 34

149281-42-5
50 suppliers
$60.00/100mg

31600-86-9
0 suppliers
inquiry

86580-59-8
14 suppliers
$160.00/100mg