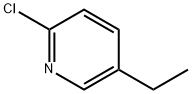
2-Chloro-5-ethyl-pyridine synthesis
- Product Name:2-Chloro-5-ethyl-pyridine
- CAS Number:90196-32-0
- Molecular formula:C7H8ClN
- Molecular Weight:141.6
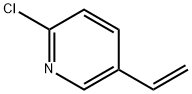
157670-28-5

90196-32-0
General procedure for the synthesis of 2-chloro-5-ethylpyridine from 2-chloro-5-vinylpyridine: 2-chloro-5-vinylpyridine (1 g, 7.2 mmol) was dissolved in 20 mL of methanol. Hydrogen was passed into the resulting solution for 2 min, followed by the addition of 1,5-cyclooctadiene(pyridine)(tricyclohexylphosphine)iridium(I) hexafluorophosphate catalyst (0.1 g). The mixture was placed in a hydrogenation reactor and reacted for 2 hours under 1 atm hydrogen. Upon completion of the reaction, the reaction solution was filtered through diatomaceous earth and concentrated. The organic phase was separated by adding 10 mL of dichloromethane and 10 mL of water to the concentrate. The organic phase was dried with anhydrous magnesium sulfate, filtered and the solvent was evaporated and concentrated to give 0.84 g of 2-chloro-5-ethylpyridine as a pale yellow liquid in 83% yield.

157670-28-5
39 suppliers
$40.00/250mg

90196-32-0
68 suppliers
$78.00/100mg
Yield:90196-32-0 83%
Reaction Conditions:
with Crabtree's catalyst;hydrogen in methanol; under 760.051 Torr; for 2 h;Time;
Steps:
3 preparation of 2-chloro-5-ethylpyridine
2-chloro-5-vinylpyridine (1g, 7.2mmol) is dissolved in 20 ml of methanol; the resulting solution is passed through hydrogen for 2 minutes, after that added 1,5-cyclooctadiene (pyridine) (tricyclohexyl-phosphine) iridium (I) hexafluorophosphate catalyst (0.1g), subsequently, the mixture is reacted in a hydrogenation reactor (1atm hydrogen) for 2 hours. The reaction solution is concentrated by filtration through diatomite, and then added 10 ml of dichloromethane and 10 ml of water. The organic phase is separated and dried over anhydrous magnesium sulfate, filtration, steaming and concentrating and then obtained 2-chloro-5-ethylpyridine 0.84g, light yellow liquid, yield 83%.
References:
CN104529881,2017,B Location in patent:Paragraph 0019; 0032; 0033

157670-28-5
39 suppliers
$40.00/250mg
1121-55-7
141 suppliers
$122.00/1g
536-78-7
209 suppliers
$25.00/5mL

90196-32-0
68 suppliers
$78.00/100mg
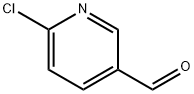
23100-12-1
274 suppliers
$6.00/1g

90196-32-0
68 suppliers
$78.00/100mg
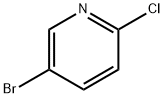
53939-30-3
484 suppliers
$5.00/1g

90196-32-0
68 suppliers
$78.00/100mg

