
2-CHLORO-5-HYDROXYBENZALDEHYDE synthesis
- Product Name:2-CHLORO-5-HYDROXYBENZALDEHYDE
- CAS Number:7310-94-3
- Molecular formula:C7H5ClO2
- Molecular Weight:156.57

100-83-4

7310-94-3
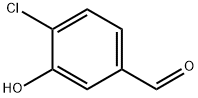
56962-12-0
3-Hydroxybenzaldehyde (1 g, 10 mmol) was used as starting material and dissolved in acetonitrile (50 mL). To this solution, p-toluenesulfonic acid was added in batches at room temperature and after stirring for 5 minutes, N-chlorosuccinimide (NCS, 1.33 g, 10 mmol) was added. The reaction mixture was continued to be stirred at room temperature for 2 hours. Upon completion of the reaction, the reaction was quenched with aqueous sodium thiosulfate, followed by dilution with ethyl acetate and saturated saline. The organic layer was separated, dried with anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography with the eluent of petroleum ether/ethyl acetate (10:1 to 5:1) to afford 2-chloro-5-hydroxybenzaldehyde (Intermediate 28a, 340 mg, 21.7% yield) and 4-chloro-3-hydroxybenzaldehyde (Intermediate 28b, 310 mg, 19.7% yield) as yellow solids, respectively.The 1H NMR (CDCl3) data of 2-chloro-5-hydroxybenzaldehyde: δ10.43 (s, 1H), 7.54-7.56 (d, 1H), 7.30-7.37 (m, 2H). 1H-NMR (CDCl3) data of 4-chloro-3-hydroxybenzaldehyde: δ10.43 (s, 1H), 7.40 (d, 1H), 7.33 (d, 1H), 7.06- 7.09 (dd, 1H).
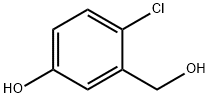
876299-47-7
34 suppliers
inquiry

7310-94-3
81 suppliers
$21.00/250mg
Yield: 81%
Reaction Conditions:
with manganese(IV) oxide in acetone for 3 h;Heating / reflux;
Steps:
27 2-Chloro-5-hydroxy-benzaldehyde
Manganese dioxide (11 g, 125 mmol) was added to a suspension of the product of preparation 26 (4 g, 25.2 mmol) in acetone (25 mL) and the mixture was heated to reflux for 3 hours. The reaction mixture was then cooled to room temperature and concentrated in vacuo. The residue was dissolved in dichloromethane:methanol, 95:5, passed through a pad of silica and concentrated in vacuo to afford the title compound as a solid in 81% yield, 3.17 g
References:
Mathias, John Paul;Millan, David Simon;Lewthwaite, Russell Andrew;Phillips, Christopher US2006/35922, 2006, A1 Location in patent:Page/Page column 31

100-83-4
648 suppliers
$5.00/10g

7310-94-3
81 suppliers
$21.00/250mg

56962-12-0
87 suppliers
$16.00/250mg

100-83-4
648 suppliers
$5.00/10g

7310-94-3
81 suppliers
$21.00/250mg

56962-12-0
87 suppliers
$16.00/250mg
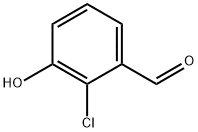
56962-10-8
111 suppliers
$21.00/250mg
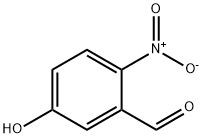
42454-06-8
215 suppliers
$8.00/1g

7310-94-3
81 suppliers
$21.00/250mg

100-83-4
648 suppliers
$5.00/10g

7310-94-3
81 suppliers
$21.00/250mg