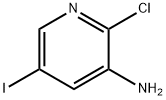
2-CHLORO-5-IODO-PYRIDIN-3-YLAMINE synthesis
- Product Name:2-CHLORO-5-IODO-PYRIDIN-3-YLAMINE
- CAS Number:426463-09-4
- Molecular formula:C5H4ClIN2
- Molecular Weight:254.46
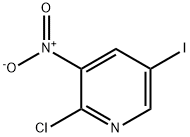
426463-05-0

426463-09-4
General procedure for the synthesis of 3-amino-2-chloro-5-iodopyridine from 2-chloro-5-iodo-3-nitropyridine: 5-iodo-3-nitro-2-chloropyridine (230 mg, 0.809 mmol), ethanol (1 mL), water (6 drops), and concentrated hydrochloric acid (0.020 mL) were stirred at room temperature for 10 minutes. Subsequently, iron powder (500 mg, 8.95 mmol) was added in small portions and the reaction mixture was heated in an oil bath at 100 °C for 20 min. After completion of the reaction, the iron powder was removed by filtration and the filtrate was washed with ethanol. The ethanol layers were combined and concentrated under reduced pressure. The crude product was purified by fast chromatography using 9:1 hexane:ethyl acetate as eluent to afford 5-iodo-3-amino-2-chloropyridine (190 mg, 92% yield) as a colorless solid with a melting point of 129 °C. 1H NMR (CDCl3) δ (ppm): 4.15 (broad single peak, 2H), 7.34 (double peak, J = 2.0 Hz, 1H), and 7.96 (double peaks, J = 2.0 Hz, 1H); 13C NMR (CDCl3) δ (ppm): 91.41, 129.67, 136.31, 140.77, 143.90. Calculated values for elemental analysis (C5H4N2Cl): C, 23.60; H, 1.58; N, 11.01; Measured values: C, 23.66; H 1.52; N, 10.98.
6332-56-5
356 suppliers
$10.00/5g

426463-09-4
66 suppliers
$10.00/100mg
Yield:-
Steps:
Multi-step reaction with 3 steps
1: 68 percent / I2; H5IO6
2: 88 percent / POCl3
3: 91 percent / SnCl2
References:
Zhang, Nanjing;Tomizawa, Motohiro;Casida, John E. [Bioorganic and medicinal chemistry letters,2003,vol. 13,# 3,p. 525 - 528]

426463-05-0
109 suppliers
$6.00/250mg

426463-09-4
66 suppliers
$10.00/100mg
4214-75-9
415 suppliers
$9.00/5g

426463-09-4
66 suppliers
$10.00/100mg
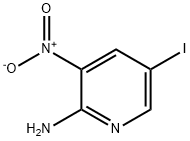
25391-57-5
119 suppliers
$11.00/1g

426463-09-4
66 suppliers
$10.00/100mg
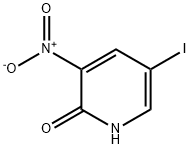
25391-59-7
63 suppliers
$9.00/100mg

426463-09-4
66 suppliers
$10.00/100mg