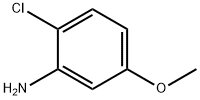
2-Chloro-5-methoxyaniline synthesis
- Product Name:2-Chloro-5-methoxyaniline
- CAS Number:2401-24-3
- Molecular formula:C7H8ClNO
- Molecular Weight:157.6
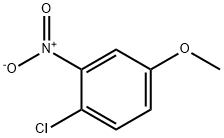
10298-80-3

2401-24-3
General procedure for the synthesis of 2-chloro-5-methoxyaniline from 4-chloro-3-nitroanisole: nitro compounds (0.6 mmol) and ethanol (2 mL) were added to a sealed tube, followed by hydrazine hydrate (2.6-6.0 mmol, cf. Table 2) and an Au/TiO2 catalyst (100 mg, 1 wt%, with 0.8 mol% [Au]). The reaction mixture was heated at 60 °C for an appropriate time under an inert atmosphere (see Table 2). The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction slurry was filtered under pressure through a short pad of diatomaceous earth and silica gel and washed using ethanol or methanol (~5 mL) to recover the loaded catalyst. The filtrate was evaporated under vacuum to give pure 2-chloro-5-methoxyaniline. The nuclear magnetic resonance hydrogen (1H NMR) and carbon (13C NMR) spectral data of the products 1a-20a were in agreement with those reported in the literature [28,30,31] and most of the products were commercially available chemicals. The Au/TiO2, Au/Al2O3 and Au/ZnO catalysts used (~1 wt% Au content) were purchased from Strem Chemicals and the average size of their Au microcrystals was about 2-3 nm.
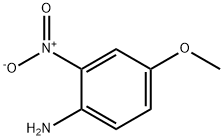
96-96-8
0 suppliers
$7.00/1g

2401-24-3
232 suppliers
$8.00/1g
Yield:-
Steps:
Multi-step reaction with 2 steps
1: (i) NaNO2, aq. HCl, (ii) CuCl
2: SnCl2, aq. HCl
References:
Munavalli,S. et al. [Bulletin de la Societe Chimique de France,1966,p. 3311 - 3318]

10298-80-3
337 suppliers
$10.00/10g

2401-24-3
232 suppliers
$8.00/1g
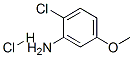
85006-21-9
164 suppliers
$17.89/1g

2401-24-3
232 suppliers
$8.00/1g