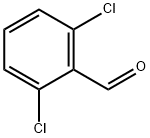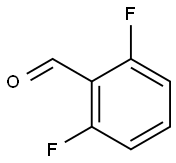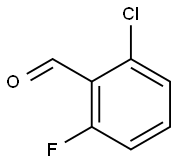
2-Chloro-6-fluorobenzaldehyde synthesis
- Product Name:2-Chloro-6-fluorobenzaldehyde
- CAS Number:387-45-1
- Molecular formula:C7H4ClFO
- Molecular Weight:158.56
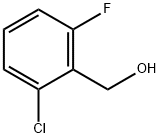
56456-50-9

387-45-1
GENERAL METHOD: A mixture of 2-chloro-6-fluorobenzyl alcohol (1.0 mmol), N-phenylglycine (0.0076 g, 0.05 mmol), CuBr2 (0.0112 g, 0.05 mmol), Na2CO3 (0.1060 g, 1.0 mmol), and TEMPO (0.0078 g, 0.05 mmol) was added to a 100 mL Schlenk tube with H2O (3.0 mL) was added to a 100 mL Schlenk tube. It was stirred vigorously for 0.5 h under reflux conditions in air. After completion of the reaction, the product was extracted with CH2Cl2 (3 x 2.0 mL). The organic phases were combined, washed with H2O (3.0 mL) and dried with anhydrous MgSO4. After vacuum concentration, the residue was purified by column chromatography to give 2-chloro-6-fluorobenzaldehyde. Isolated yield: 0.1080 g (90%).
Yield:437-81-0 81% ,387-45-1 10%
Reaction Conditions:
with potassium fluoride;anhydrous tetramethylammonium chloride in dimethyl sulfoxide at 60 - 160; under 5.25053 Torr; for 6 h;Inert atmosphere;Temperature;Solvent;
Steps:
5; 10; 12; 15 Example 10
32.0 g (550 mmol) of spray-dried potassium fluoride and 2.7 g (25 mmol) of tetramethylammonium chloride were added to a 500 mL four-necked eggplant flask, the temperature was raised to 60 °C., the pressure was reduced to 0.7 kPa, and the pressure was reduced for 1 hour. Dehydration was performed. After dehydration under reduced pressure, 43.8 g (250 mmol) of 2,6-dichlorobenzaldehyde and 62.5 g (800 mmol) of dimethyl sulfoxide were charged under a nitrogen atmosphere, the temperature was raised to 160 °C., and the reaction was carried out for 6 hours. When the reaction solution 6 hours after the reaction was analyzed by gas chromatography, 2-fluoro-6-chlorobenzaldehyde was produced in a yield of 10%, and 2,6-difluorobenzaldehyde was produced in a yield of 81%. rice field. The conversion rate of 2,6-dichlorobenzaldehyde was 99%. To the reaction solution obtained above, 100 g of ion-exchanged water and 100 g of toluene were added, extraction was performed, and the mixture was separated into an organic layer and an aqueous layer. Further, the operation of extracting the separated aqueous layer with 100 g of toluene was repeated twice. 250 g of the water layer separated by the above operation was subjected to Helipack No.1 was charged into the bottom of a distillation column (inner diameter 10 mm, filling height 100 mm, theoretical plate number 5) packed and distilled under reduced pressure.In the vacuum distillation, the column bottom temperature was heated to 90 °C. under a pressure of 5 kPa, water was distilled off, and then the pressure was gradually reduced to 0.6 kPa to recover dimethyl sulfoxide in a yield of 84%. The water content in the recovered dimethyl sulfoxide was 0.1% by weight, and the area% of dimethyl sulfoxide analyzed by GC was 99.9%.
References:
WO2022/70992,2022,A1 Location in patent:Paragraph 0051; 0056; 0058; 0059; 0063; 0064

56456-50-9
179 suppliers
$12.00/5g

387-45-1
385 suppliers
$9.00/1g
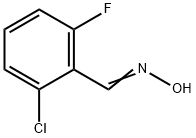
443-33-4
83 suppliers
$15.00/1g

387-45-1
385 suppliers
$9.00/1g
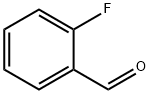
446-52-6
435 suppliers
$6.00/25g

387-45-1
385 suppliers
$9.00/1g
![Acetamide, N-[(2-chloro-6-fluorophenyl)methyl]-](/CAS/20210305/GIF/661452-15-9.gif)
661452-15-9
0 suppliers
inquiry

387-45-1
385 suppliers
$9.00/1g