
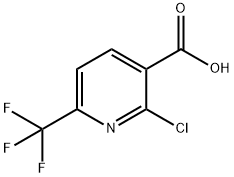
(2-chloro-6-(trifluoromethyl)pyridin-3-yl)methanol synthesis
- Product Name:(2-chloro-6-(trifluoromethyl)pyridin-3-yl)methanol
- CAS Number:917396-39-5
- Molecular formula:C7H5ClF3NO
- Molecular Weight:211.57
280566-45-2
233 suppliers
$9.00/100mg

917396-39-5
36 suppliers
$65.00/10mg
Yield:917396-39-5 100%
Reaction Conditions:
with borane-THF in tetrahydrofuran at 0 - 20; for 20 h;Inert atmosphere;
Steps:
6 8-(Trifluoromethyl)-2,3,4,5-tetrahydro-1H-pyrido[2,3-e][1,4]diazepine (6)
A solution of borane-THF complex in THF (0.9 M, 70 mL, 63 mmol) was added to a solution of 2-chloro-6-(trifluoromethyl)pyridine-3-carboxylic acid (5.10 g, 22.1 mmol) in THF (100 mL) under N2 atmosphere at 0 °C, then the mixture was gradually raised to room temperature and stirred for 20 h.
After addition of methanol (10 mL), the reaction mixture was diluted with AcOEt (100 mL), washed with brine, dried (Na2SO4), concentrated under reduced pressure and the residue was purified by silica gel column chromatography (hexane/AcOEt = 1:1) to obtain the alcohol (4.66 g, quant.) as a colorless oil. 1H NMR (CDCl3) δ: 8.12 (1H, d, J = 7.8 Hz), 7.69 (1H, d, J = 7.8 Hz), 4.87 (2H, d, J = 5.5 Hz), 2.06 (1H, t, J = 5.5 Hz).
The obtained alcohol (42.4 g, 201 mmol) was dissolved in ethylenediamine (161 ml, 1.20 mol), and the reaction mixture was stirred at 120 °C for 12 h.
The cooled mixture was diluted with brine, extracted with AcOEt (300 mL * 3).
The organic layer was dried over Na2SO4, concentrated and purified by silica gel column chromatography (hexane/AcOEt = 3:1) to provide the amino alcohol (29.4 g, 62%) as a colorless oil. 1H NMR (CDCl3) δ: 7.33 (1H, d, J = 7.4 Hz), 6.85 (1H, d, J = 7.4 Hz), 6.03 (1H, br s), 4.59 (2H, s), 3.72 (2H, q, J = 5.9 Hz), 3.48 (2H, t, J = 6.3 Hz). MnO2 (98.8 g, 1.00 mol) was added to a solution of the amino alcohol (29.4 g, 125 mmol) in CH2Cl2/MeOH (700 mL/100 mL), and the reaction mixture was stirred at room temperature for 2 h.
The mixture was filtered through a Celite pad, concentrated to provide the imine (20.4 g, 76%).
1H NMR (CDCl3) δ: 8.27 (1H, t, J = 1.6 Hz), 7.75 (1H, d, J = 7.8 Hz), 7.04 (1H, d, J = 7.8 Hz), 5.99 (1H, br s), 4.14-4.11 (2H, m), 3.49-3.46 (2H, m).
10% Pd/C (8.00 g) was added to a solution of the imine (20.4 g, 94.8 mmol) in AcOEt (250 mL), and the reaction mixture was stirred under H2 atmosphere at room temperature for 4 h.
The mixture was filtered through a Celite pad, concentrated, and purified by silica gel column chromatography (MeOH/CH2Cl2 = 1:8) to provide the diazepine 6 (16.9 g, 82%) as a yellow oil. 1H NMR (CDCl3) δ: 7.46 (1H, d, J = 7.4 Hz), 7.07 (1H, d, J = 7.4 Hz), 5.11 (1H, s), 3.91 (2H, br s), 3.49 (1H, s), 3.28-3.25 (2H, m), 3.11-3.09 (2H, m)
References:
Matsufuji, Tetsuyoshi;Shimada, Kousei;Kobayashi, Shozo;Ichikawa, Masanori;Kawamura, Asuka;Fujimoto, Teppei;Arita, Tsuyoshi;Hara, Takashi;Konishi, Masahiro;Abe-Ohya, Rie;Izumi, Masanori;Sogawa, Yoshitaka;Nagai, Yoko;Yoshida, Kazuhiro;Abe, Yasuyuki;Kimura, Takako;Takahashi, Hisashi [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 1,p. 89 - 104]
39890-95-4
349 suppliers
$5.00/1g

917396-39-5
36 suppliers
$65.00/10mg