2-chloro-N-[2-(trifluoromethyl)phenyl]benzamide synthesis
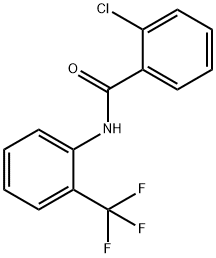
- Product Name:2-chloro-N-[2-(trifluoromethyl)phenyl]benzamide
- CAS Number:3873-78-7
- Molecular formula:C14H9ClF3NO
- Molecular Weight:299.68
Yield:3873-78-7 73 %
Reaction Conditions:
with tetraethylammonium perchlorate in water;acetonitrile at 25;Sonication;
Steps:
21 Example 21
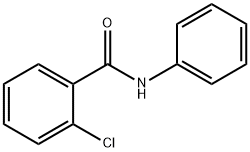
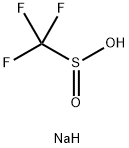
0.2 mmol of starting material 2-chloro-N-phenylbenzamide,0.4 mmol of sodium trifluoromethyl sulfinate, 0.1 mmolTetraethylammonium perchlorate was dissolved in a mixed solution of 4 mL of acetonitrile and 2 mL of water.The reaction mixture was sonicated at 25°C for 30 minutes to aid dissolution. at ambient temperature,At a constant current of 9mA, theAt a flow rate of 0.025mL/min, it was injected into a microchannel reactor with a carbon sheet electrode as the positive electrode and a platinum-plated electrode as the negative electrode.After the reaction solution was collected, it was washed with 50 mL of saturated sodium bicarbonate solution,50mL ethyl acetate extracted 3 times, the organic phase was dried over anhydrous sodium sulfate,The filtrate was concentrated under reduced pressure and separated by column chromatography (petroleum ether: ethyl acetate = 8:1 volume ratio) to obtain the product ortho-trifluoromethyl-substituted aniline product 5 in a yield of 90%.
References:
CN113445066,2022,B Location in patent:Paragraph 0084-0088