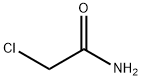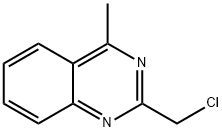
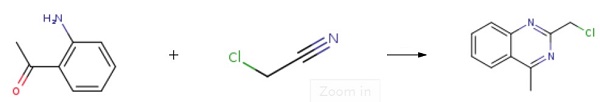
2-(chloromethyl)-4-methylquinazoline synthesis
- Product Name:2-(chloromethyl)-4-methylquinazoline
- CAS Number:109113-72-6
- Molecular formula:C10H9ClN2
- Molecular Weight:192.64
Yield:109113-72-6 94.26%
Reaction Conditions:
Stage #1: 2-aminoacetophenonewith phosphoric acid in ethanol at 20;
Stage #2: Chloroacetamide in ethanol;Reflux;Reagent/catalyst;Solvent;
Steps:
1-6 Example 1
Put 13.52g (0.1mol, M=135.17) of o-aminoacetophenone into the round bottom flask, Add 80ml of absolute ethanol and stir to dissolve, add 11.76g of catalyst H3PO4 (0.12mol, M=98), slowly add dropwise dissolved in 20ml absolute ethanol at room temperature A solution of 10.29g (0.11mol, M=93.51) of 2-chloroacetamide, after dripping, The reaction was refluxed for 45h, cooled and filtered, and the filtrate was washed with saturated brine, The solvent was evaporated and the organic phase was extracted with ethyl acetate and dried with anhydrous sodium sodium sulfate. Concentrate and dry in vacuo to obtain 18.16 g of solid intermediate II with a yield of 94.26%. The purity is 99.9%, and the maximum single impurity is 0.01%.
References:
CN112679500,2021,A Location in patent:Paragraph 0036-0048
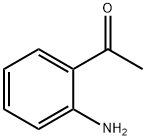
551-93-9
502 suppliers
$6.00/5g

107-14-2
296 suppliers
$15.00/25g

109113-72-6
477 suppliers
$9.00/1g

107-14-2
296 suppliers
$15.00/25g

109113-72-6
477 suppliers
$9.00/1g
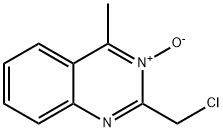
6640-59-1
1 suppliers
inquiry

109113-72-6
477 suppliers
$9.00/1g
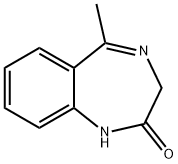
70656-87-0
12 suppliers
inquiry

109113-72-6
477 suppliers
$9.00/1g