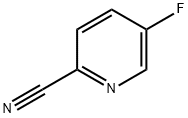
2-Cyano-5-fluoropyridine synthesis
- Product Name:2-Cyano-5-fluoropyridine
- CAS Number:327056-62-2
- Molecular formula:C6H3FN2
- Molecular Weight:122.1
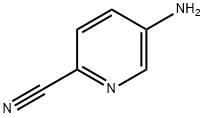
55338-73-3

327056-62-2
General procedure for the synthesis of 2-cyano-5-fluoropyridine from 3-amino-6-cyanopyridine: Sodium nitrite (8.7 g, 126 mmol) was added in batches to ice-salt-bath-cooled 5-amino-pyridine-2-carbonitrile (10.03 g, 84.2 mmol) dissolved in a solution of 70% hydrogen fluoride-pyridine (Aldrich, 100 g, 3.5 mol HF) ( Note: the reaction was carried out in a special reaction flask). The reaction solution was dark red in color, and after stirring in an ice-salt bath for 45 min, the ice bath was removed and the mixture continued to be stirred at room temperature for 30 min, followed by heating at 80 °C for 1.5 h. The reaction was carried out in a special reaction flask. Upon completion of the reaction, the reaction mixture was quenched by slowly pouring it into an ice/water mixture (about 400 g), extracted with dichloromethane (6 x 150 mL), the organic phase was dried over anhydrous magnesium sulfate, filtered and the solvent was concentrated under reduced pressure. The resulting crude product (10.08 g, 98% yield, orange solid) was pure enough for subsequent use and did not require further purification. The product was characterized by 1H NMR (CDCl3, 300 MHz): δ= 8.61 (d, 1H), 7.78 (m, 1H), 7.58 (m, 1H).GC-MS showed the molecular ion peak M+ 122.
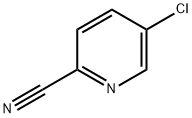
89809-64-3
269 suppliers
$14.00/5g

327056-62-2
273 suppliers
$5.00/1g
Yield:327056-62-2 425 mg (48%)
Reaction Conditions:
with potassium fluoride in 1-methyl-pyrrolidin-2-one
Steps:
1 5-Fluoropyrid-2-ylamidoxime
A mixture of 2-cyano-5-chloropyridine (1 g, 7.22 mmol) and potassium fluoride (1.26 g, 21.68 mmol) in 1-methyl-2-pyrrolidinone (25 mL) was heated at reflux for 18 hours. After cooling, the reaction was diluted with ethyl acetate and extracted with water and brine. The organic solvents were then removed in vacuo. Silica gel chromatography of the residue afforded 425 mg (48%) of 2-cyano-5-fluoropyridine.
References:
Wagenen, Bradford Van;Stormann, Thomas M.;Moe, Scott T.;Sheehan, Susan M.;McLeod, Donald A.;Smith, Daryl L.;Isaac, Methvin Benjamin;Slassi, Abdelmalik US2003/55085, 2003, A1

55338-73-3
211 suppliers
$26.00/100mg

327056-62-2
273 suppliers
$5.00/1g
557-21-1
106 suppliers
$12.00/5g
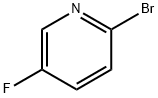
41404-58-4
410 suppliers
$5.00/1g

327056-62-2
273 suppliers
$5.00/1g

41404-58-4
410 suppliers
$5.00/1g
557-21-1
106 suppliers
$12.00/5g

327056-62-2
273 suppliers
$5.00/1g
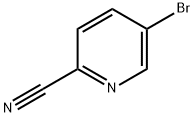
97483-77-7
575 suppliers
$10.00/5g

327056-62-2
273 suppliers
$5.00/1g