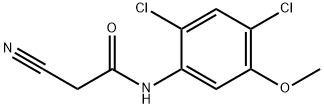
2-CYANO-N-(2,4-DICHLORO-5-METHOXYPHENYL) ACETAMIDE synthesis
- Product Name:2-CYANO-N-(2,4-DICHLORO-5-METHOXYPHENYL) ACETAMIDE
- CAS Number:846023-24-3
- Molecular formula:C10H8Cl2N2O2
- Molecular Weight:259.09
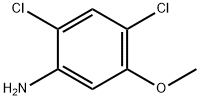
98446-49-2
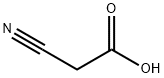
372-09-8

846023-24-3
Example 4 Synthesis of 2-cyano-N-(2,4-dichloro-5-methoxyphenyl)acetamide: 2,4-dichloro-5-methoxyaniline (5.00 g, 26 mmol) was mixed with cyanoacetic acid (2.28 g, 26.8 mmol) at 50 °C. Tetrahydrofuran was added to the mixture until complete dissolution. Subsequently, the solution was heated to reflux and 1,3-diisopropylcarbodiimide (4.2 mL, 26.8 mmol) was added slowly and dropwise. After 30 minutes of reaction, the reaction mixture was cooled to about 15 °C in an ice bath. The solid product formed was collected by filtration and washed with tetrahydrofuran. The filtrate was slowly poured into water and stirred for 30 minutes. The white solid product was collected by filtration again, washed with water and dissolved in 500 mL of ethyl acetate. The resulting solution was dried over anhydrous sodium sulfate and subsequently concentrated under vacuum to afford 5.9 g (88% yield) of the target compound 2-cyano-N-(2,4-dichloro-5-methoxyphenyl)acetamide as a white solid with a melting point of 180-181 °C.1H NMR (400 MHz, DMSO-d6) δ 3.84 (s, 3H), 4.02 (s, 2H) 7.58 (s, 1H), 7.66 (s, 1H), 10.00 (s, 1H); mass spectra (electrospray ionization) m/z 257.0, 259.0 ([M-H]-). Elemental analysis (C10H8Cl2N2O2) calculated values: C, 46.36; H, 3.11; N, 10.81. measured values: C, 46.25; H, 3.10; N, 10.85.

98446-49-2
259 suppliers
$6.00/1g

372-09-8
368 suppliers
$20.00/25g

846023-24-3
124 suppliers
inquiry
Yield:846023-24-3 88%
Reaction Conditions:
with diisopropyl-carbodiimide in tetrahydrofuran; for 0.5 h;Product distribution / selectivity;Heating / reflux;
Steps:
4 2-Cyano-N-(2,4-dichloro-5-methoxyphenyl)acetamide
EXAMPLE 4 2-Cyano-N-(2,4-dichloro-5-methoxyphenyl)acetamide 2,4-Dichloro-5-methoxyaniline (5.00 g, 26 mmol) and cyanoacetic acid (2.28 g, 26.8 mmol) were mixed in 50 mL of tetrahydrofuran until a solution formed. This solution was heated to reflux and 1,3-diisopropylcarbodiimide (4.2 mL, 26.8 mmol) was added dropwise. After 30 minutes the mixture was cooled to ~15° C. in an ice-bath. The solid was collected by filtration and washed with tetrahydrofuran. The filtrate was slowly poured into water and stirred for 30 minutes. The white solid was collected by filtration, washed with water, and was then dissolved in 500 mL of ethyl acetate. The solution was dried over sodium sulfate and concentrated in vacuo to give 5.9 g (88%) of 2-cyano-N-(2,4-dichloro-5-methoxyphenyl)acetamide as a white solid, mp 180-181° C.; 1H NMR (400 MHz, DMSO-d6) δ 3.84 (s, 3H), 4.02 (s, 2H), 7.58 (s, 1 H), 7.66 (s, 1 H), 10.00 (s, 1 H); MS (ES) m/z257.0, 259.0 (M-H)- Analysis for C10H8Cl2N2O2; Calcd: C, 46.36; H, 3.11; N, 10.81. Found: C, 46.25; H, 3.10; N, 10.85.
References:
US2005/43537,2005,A1 Location in patent:Page/Page column 3; 6

98446-49-2
259 suppliers
$6.00/1g

372-09-8
368 suppliers
$20.00/25g
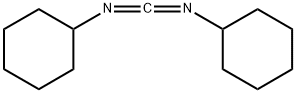
538-75-0
880 suppliers
$15.00/25g

846023-24-3
124 suppliers
inquiry
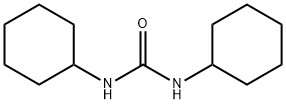
2387-23-7
224 suppliers
$8.00/5g
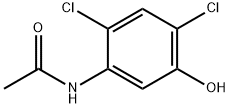
67669-19-6
71 suppliers
$9.00/250mg

846023-24-3
124 suppliers
inquiry
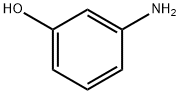
591-27-5
545 suppliers
$11.00/10g

846023-24-3
124 suppliers
inquiry