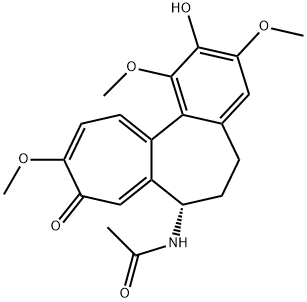
2-DEMETHYLCOLCHICINE synthesis
- Product Name:2-DEMETHYLCOLCHICINE
- CAS Number:7336-36-9
- Molecular formula:C21H23NO6
- Molecular Weight:385.41
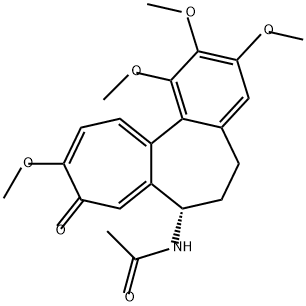
64-86-8
637 suppliers
$15.00/100mg

7336-36-9
14 suppliers
inquiry
Yield:7336-36-9 53%
Reaction Conditions:
with sulfuric acid at 60; for 7 h;
Steps:
2-Demethylcolchicine (7)
Concentrated H2SO4 (98%, 7 mL) was added to colchicine 6 (1.0 g, 2.5 mmol). The reaction mixture was stirred for 7 h at 60C. The completion of the reaction was monitored by TLC using CH2Cl2-95% EtOH (9.5 : 1.5) as the eluent. The resulting yellow solution was poured onto ice (100 g), a 2 NaOH solution was added to pH 5, and the mixture was extracted with CH2Cl2 (3×50 mL). The organic layer was dried over Na2SO4. After the removal of the solvent, the product was purified by silica gel column chromatography usinga CH2Cl2-95% EtOH mixture (9.5 : 1.5) as the eluent. The product was obtained in a yield of 0.51 g (53%) as yellow crystals,m.p. 176 C. 1H NMR (400 MHz, DMSO-d6), δ: 8.63 (s, 1 H,OH); 8.55 (d, 1 H, NH, J = 7.5 Hz); 7.13 (s, 1 H, H(8)); 7.10(d, 1 H, H(11), J = 10.7 Hz); 7.01 (d, 1 H, H(12), J = 10.7 Hz);6.66 (s, 1 H, H(4)); 4.34 (dt, 1 H, H(7), J = 12.1 Hz, J = 7.0 Hz);3.87 (s, 3 H, OC(10)H3); 3.81 (s, 3 H, OC(3)H3); 3.45 (s, 3 H,OC(1)H3); 2.45-2.58 (m, 1 H, H(6)); 2.13-2.24 (m, 1 H, H(6));
References:
Fedorov, A. Yu.;Kuzmina, N. S.;Malysheva, Yu. B.;Mol’kova, E. A.;Otvagin, V. F.;Shchegravina, E. S.;Svirshchevskaya, E. V.;Zaburdaeva, E. A. [Russian Chemical Bulletin,2022,vol. 71,# 3,p. 564 - 571][Izv. Akad. Nauk, Ser. Khim.,2022,# 3,p. 564 - 571]

64-86-8
637 suppliers
$15.00/100mg

7336-36-9
14 suppliers
inquiry
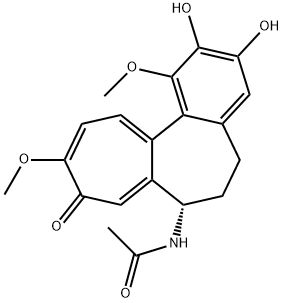
57866-21-4
3 suppliers
inquiry
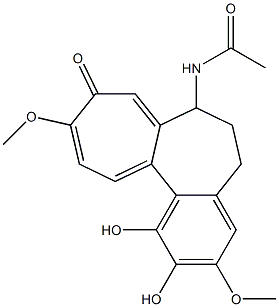
78231-83-1
0 suppliers
inquiry