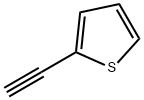
2-Ethynylthiophene synthesis
- Product Name:2-Ethynylthiophene
- CAS Number:4298-52-6
- Molecular formula:C6H4S
- Molecular Weight:108.16
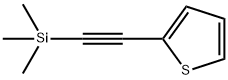
40231-03-6

4298-52-6
The general procedure for the synthesis of 2-trimethylsilylthienylthiophene from 2-trimethylsilylthienylthiophene was as follows: 2-[(trimethylmethylsilyl)ethynyl]thiophene (1 mmol), an inorganic base (potassium tert-butoxide, sodium tert-butoxide, potassium hydroxide, sodium hydroxide, or potassium trimethylsilyl, sodium trimethylsilyl, 0.05 mmol), and 2 mL of DMA or DMSO solvent were added in turn to a 10-mL in a sealed tube. The reaction mixture was placed in an oil bath at 60 °C and stirred for 6 h, during which the reaction progress was monitored by TLC. Upon completion of the reaction, an equal volume of homotrimethylene or n-undecane was added to the crude product. The exact yield of the product was determined by GC and GC-MS. The yields of the products were 59%, 66%, 75%, 77%, 92%, and 85% when DMSO was used as the reaction solvent and potassium tert-butanol, sodium tert-butanol, potassium hydroxide, sodium hydroxide, or potassium trimethylmethylsilyl, sodium trimethylmethylsilyl were used as the catalysts, respectively. When DMA was used as the reaction solvent, with the same catalyst, the yields of the products were 51%, 60%, 67%, 70%, 81%, 72%, respectively.
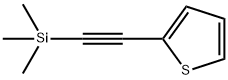
40231-03-6
100 suppliers
$45.00/1g

4298-52-6
166 suppliers
$24.00/1g
Yield:4298-52-6 92%
Reaction Conditions:
with potassium trimethylsilonate in dimethyl sulfoxide at 60; under 760.051 Torr; for 6 h;Catalytic behavior;Sealed tube;Reagent/catalyst;Solvent;
Steps:
33
2-[(trimethylsilyl)ethynyl]thiophene (1 mmol), inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilylate (sodium) (0.05 mmol), 2 mL of DMA or DMSO solvent was sequentially added to a 10 mL sealed tube and heated and stirred in a 60° C. oil bath for 6 hours. The progress of the reaction was followed by TLC. The reaction was completed and an equivalent of mesitylene or n-undecane was added to the crude product. The exact yield of the product was determined by GC and GC-MS. According to GC and GC-MS, when DMSO is used as a reaction solvent, inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilylate (sodium) is used as a catalyst, and the yields of the products are as follows: 59%, 66%, 75%, 77%, 92%, 85%. When DMA was used as the reaction solvent, the inorganic potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilylate (sodium) was used as a catalyst, and the yield of the product was: 51%, 60%, respectively. 67%, 70%, 81%, 72%.
References:
Taizhou University;Yao Wubing CN107459438, 2017, A Location in patent:Paragraph 0080; 0081
77295-66-0
1 suppliers
inquiry

4298-52-6
166 suppliers
$24.00/1g

133844-84-5
7 suppliers
inquiry

4298-52-6
166 suppliers
$24.00/1g
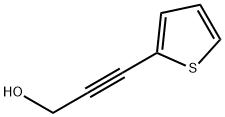
1194-13-4
7 suppliers
inquiry

4298-52-6
166 suppliers
$24.00/1g
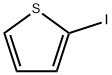
3437-95-4
250 suppliers
$6.00/5g
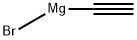
4301-14-8
126 suppliers
$55.20/100ml

4298-52-6
166 suppliers
$24.00/1g