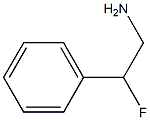
2-fluoro-2-phenylethan-1-amine synthesis
- Product Name:2-fluoro-2-phenylethan-1-amine
- CAS Number:55601-20-2
- Molecular formula:C8H10FN
- Molecular Weight:139.17
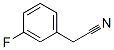
10036-43-8
27 suppliers
inquiry

55601-20-2
8 suppliers
inquiry
Yield:55601-20-2 49%
Reaction Conditions:
Stage #1: α-(Fluorophenyl)acetonitrilewith borane-THF in tetrahydrofuran at 0; for 0.666667 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;ethanol;
Steps:
4.1.2.3. Preparation of 2-fluoro-2-phenylethylamine: 17
Compound 17 was prepared following a literature methodrefPreviewPlaceHolder41 with modifications. Briefly, to a stirred solution of mandelonitrile (1.33 g, 1.0 mmol, 1.0 equiv) in dry DCM (5 mL) at 0 °C was added diethylaminosulfure trifluoride (DAST) (1.77 g, 1.1 mmol, 1.1 equiv) in DCM (2 mL). The resulting mixture was stirred at 0 °C and stirred for 30 min, diluted with DCM (15 mL), and treated with saturated NaHCO3. The organic layer was collected and dried over MgSO4. After removal of the solvent under vacuum, the crude product was chromatographed on a silica gel column and eluted with 10% EtOAc/hexane to afford α-fluorophenylacetonitrile as yellow oil (0.88 g) in 65% yield. The 1H NMR spectrum was consistent with the literature,refPreviewPlaceHolder41 and 19F NMR showed a doublet at -167.4 ppm with J = 48 Hz. Finally, the cyano-group was reduced to the corresponding amine by treatment with 1 M borane-THF solution in THF at 0 °C for 40 min and quenched the reaction mixture with ethanolic hydrochloric acid, then concentrated under reduced pressure. The desired 2-fluorophenylethyl amine was triturated with CH3CN and then recrystallized from ethanol/acetonitrile to afford 17 as colorless crystals in 49% yield. 1H NMR (CDCl3) δ: 7.50 (m, 5H, aromatic), 5.8 (dddd, J = 48.0, 8.4, 7.2, 4.5, 3.0 Hz, 1H, benzylic), 3.50 (m, 2H, methylene). 19F NMR (CDCl3) δ: -183 (dm). MS: 154 (M+H).
References:
Turkman, Nashaat;Shavrin, Aleksander;Ivanov, Roman A.;Rabinovich, Brian;Volgin, Andrei;Gelovani, Juri G.;Alauddin, Mian M. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 18,p. 5698 - 5707] Location in patent:experimental part
1499-00-9
24 suppliers
$275.00/0.25g

55601-20-2
8 suppliers
inquiry

115046-20-3
0 suppliers
inquiry

55601-20-2
8 suppliers
inquiry

2932-58-3
8 suppliers
inquiry

55601-20-2
8 suppliers
inquiry
70789-41-2
0 suppliers
inquiry

55601-20-2
8 suppliers
inquiry