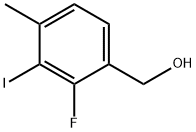
(2-FLUORO-3-IODO-4-METHYLPHENYL)METHANOL synthesis
- Product Name:(2-FLUORO-3-IODO-4-METHYLPHENYL)METHANOL
- CAS Number:1067904-88-4
- Molecular formula:C8H8FIO
- Molecular Weight:266.05
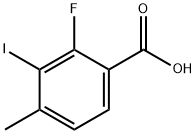
882679-89-2
16 suppliers
$187.94/1g

1067904-88-4
8 suppliers
inquiry
Yield: 97%
Reaction Conditions:
Stage #1:2-fluoro-3-iodo-4-methylbenzoic acid with Trimethyl borate in tetrahydrofuran at 20; for 0.25 h;Inert atmosphere;
Stage #2: with dimethylsulfide borane complex in tetrahydrofuran at 0 - 20; for 3 h;Inert atmosphere;
Stage #3: with methanol in tetrahydrofuran for 0.5 h;
Steps:
10.d
d) (2-Fluoro-3-iodo-4-methylphenyl)methanol; Trimethyl borate (6.5 mL, 0.06 moi) was added to a stirred solution of 2-fluoro-3-iodo-4- methylbenzoic acid (Intermediate 10c, 15.78 g, 0.06 moi) in tetrahydrofuran (80 mL) under an argon atmosphere and the mixture was stirred at room temperature. After 15 minutes, a solution of borane dimethyl sulphide (11.50 mL, 0.12 moi) in tetrahydrofuran (10 mL) was added to the reaction mixture at 0 °C. The reaction was stirred at room temperature for 3 hours. After that, methanol (5 mL) was added slowly to the mixture. After 30 minutes, the solvent was evaporated, and ethyl acetate was added to the residue, the organic layer was washed with saturated aqueous sodium hydrogen carbonate solution and water, dried (Na2S04) and evaporated to give the title compound (14.60 g, 97%) as a white solid.1 H NMR (300 MHz, CHLOROFORM-d) δ ppm 2.47 (s, 3 H) 4.74 (s, 2 H) 7.05 (d, J=7.97 Hz, 1 H) 7.28 (t, J=7.55 Hz, 1 H)
References:
ALMIRALL, S.A.;AIGUADE BOSCH, José;CARRANCO MORUNO, Inés WO2011/57757, 2011, A1 Location in patent:Page/Page column 41
85070-67-3
216 suppliers
$5.00/1g

1067904-88-4
8 suppliers
inquiry
909185-86-0
16 suppliers
inquiry

1067904-88-4
8 suppliers
inquiry
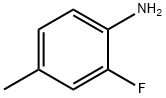
452-80-2
285 suppliers
$6.00/1g

1067904-88-4
8 suppliers
inquiry