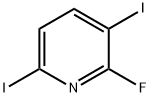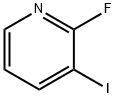
2-Fluoro-3-iodopyridine synthesis
- Product Name:2-Fluoro-3-iodopyridine
- CAS Number:113975-22-7
- Molecular formula:C5H3FIN
- Molecular Weight:222.99
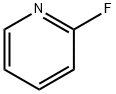
372-48-5

113975-22-7
General procedure for the synthesis of 2-fluoro-3-iodopyridine from 2-fluoropyridine: LDA (25.8 mL of a 2M THF solution, 51.5 mmol) was added slowly and dropwise to an anhydrous THF (50 mL) solution of 2-fluoropyridine (5.0 g, 51.5 mmol) at -78 °C. The reaction mixture was stirred continuously at -78 °C for 1 hour. Subsequently, a THF (30 mL) solution of I2 (13.07 g, 51.5 mmol) was added dropwise to the reaction system at the same temperature. After the reaction mixture continued to be stirred at -78 °C for 3 h, the reaction was quenched by the addition of H2O (10 mL) and slowly warmed to room temperature. Na2SO3 (50 mL, 2 M solution in water) was added to remove excess iodine. The organic layer was separated, washed with brine, dried over anhydrous Na2SO4 and then concentrated under reduced pressure to give the crude product. The crude product was purified by column chromatography to afford 2-fluoro-3-iodopyridine (9.6 g, 83.6% yield) as a white solid. lC/MS analysis: m/z (M++H) = 224.

372-48-5
385 suppliers
$5.00/5g

113975-22-7
314 suppliers
$7.00/1g
Yield:113975-22-7 92%
Reaction Conditions:
Stage #1:2-fluoropyridine with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 4 h;
Stage #2: with iodine in tetrahydrofuran;hexane at -78; for 1 h;
Steps:
1.a
(a) 222 mL of n-butyllithium (1.57 mol/L hexane solution) was dropwise added at -20°C to a solution having 34.2 g (340 mmol) of diisopropylamine dissolved in 400 mL of tetrahydrofuran, followed by stirring for 1 hour. The solution was cooled to -78°C, a solution having 32.0 g (330 mmol) of 2-fluoropyridine dissolved in 50 mL of tetrahydrofuran was added thereto, followed by stirring for 4 hours, and a solution having 87.1 g (341 mmol) of iodine dissolved in 150 mL of tetrahydrofuran was added thereto, followed by stirring for 1 hour. 200 mL of water was added to the mixture to terminate the reaction, and tetrahydrofuran was distilled off under reduced pressure. Extraction with ethyl ether was carried out, the organic layer was dried over anhydrous sodium sulfate and subjected to filtration, and the solvent was distilled off under reduced pressure, to obtain 67.4 g (crude yield 92%) of a crude product of 2-fluoro-3-iodopyridine.1H-NMR(CDCl3, 400 MHz) : δ (ppm) = 6.91-6.88(m,1H), 8.08-8.12(m,2H)
References:
ISHIHARA SANGYO KAISHA, LTD. EP1559320, 2005, A1 Location in patent:Page/Page column 6
78607-36-0
370 suppliers
$14.00/1g

113975-22-7
314 suppliers
$7.00/1g

372-48-5
385 suppliers
$5.00/5g

113975-22-7
314 suppliers
$7.00/1g
22282-70-8
274 suppliers
$8.00/1g
36178-05-9
235 suppliers
$15.00/5g

113975-22-7
314 suppliers
$7.00/1g