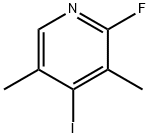
2-Fluoro-4-iodo-3,5-dimethylpyridine synthesis
- Product Name:2-Fluoro-4-iodo-3,5-dimethylpyridine
- CAS Number:1429510-62-2
- Molecular formula:C7H7FIN
- Molecular Weight:251.04
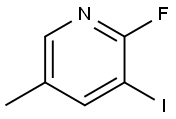
153034-78-7
172 suppliers
$11.00/250mg

74-88-4
357 suppliers
$15.00/10g

1429510-62-2
31 suppliers
inquiry
Yield:1429510-62-2 86.9 g
Reaction Conditions:
Stage #1: 2-Fluoro-3-iodo-5-methylpyridinewith lithium diisopropyl amide in tetrahydrofuran;hexane at -74 - -72; for 1.5 h;
Stage #2: methyl iodide in tetrahydrofuran;hexane at -74 - -70; for 2 h;
Steps:
29.2 (2)
Synthesis of 2-fluoro-4-iodo-3,5-dimethylpyridine
(2)
Synthesis of 2-fluoro-4-iodo-3,5-dimethylpyridine
Diisopropylamine (88 mL) was added to THF (1.2 L), and the mixture was cooled to -18° C. in a nitrogen atmosphere.
A 2.69 M solution of n-butyllithium in hexane (215 mL) was added dropwise to the solution.
After completion of the dropwise addition, the mixture was warmed to -5° C. with stirring over 30 minutes.
The reaction mixture was cooled to -72° C. A solution of 2-fluoro-3-iodo-5-methylpyridine (109.69 g) in TIE (240 mL) was added dropwise to the reaction mixture.
The reaction mixture was stirred at -74° C. for 1.5 hours.
A solution of methyl iodide (36 mL) in THF (160 mL) was added dropwise to the reaction mixture.
The reaction mixture was stirred at -70° C. to -74° C. for two hours.
After completion of the reaction, water (200 mL) was added to the reaction mixture at the same temperature.
The mixture was stirred at the same temperature for two minutes.
The reaction mixture was returned to room temperature, and water (1.2 L) was then added.
The mixed solution was stirred for three minutes.
Water (300 mL) was further added.
The mixture was extracted with MTBE (1.2 L).
The organic layer was washed with brine (500 mL).
The combined aqueous layers were extracted with MTBE (1 L).
The combined organic layers were dried over anhydrous magnesium sulfate.
The desiccant was removed by filtration, and the filtrate was concentrated under reduced pressure. n-heptane (100 mL) was added to the residue, followed by cooling.
The precipitated solid was collected by filtration.
The residue was washed with n-heptane.
The filtrate was cooled, and the precipitated solid was collected by filtration.
This operation was repeated twice to give the title compound (86.9 g).
1H-NMR (400 MHz, CDCl3) δ (ppm): 2.39-2.40 (m, 6H), 7.80-7.82 (m, 1H).
ESI-MS m/z 252 [M+H]+
References:
US2013/143907,2013,A1 Location in patent:Paragraph 0429; 0430; 0431
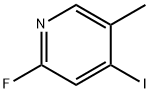
153034-94-7
199 suppliers
$8.00/250mg

74-88-4
357 suppliers
$15.00/10g

1429510-62-2
31 suppliers
inquiry
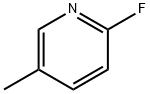
2369-19-9
279 suppliers
$6.00/1g

1429510-62-2
31 suppliers
inquiry