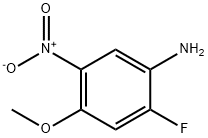
2-fluoro-4-methoxy-5-nitrophenylamine synthesis
- Product Name:2-fluoro-4-methoxy-5-nitrophenylamine
- CAS Number:1569986-91-9
- Molecular formula:C7H7FN2O3
- Molecular Weight:186.14
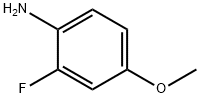
458-52-6
130 suppliers
$10.00/1g

1569986-91-9
30 suppliers
inquiry
Yield: 69%
Reaction Conditions:
with sulfuric acid;potassium nitrate at 0; for 2 h;
Steps:
Synthesis of Intermediate III:
KNO3 (35.8 g, 354 mmol) is slowly added to a solution of Compound a (50.0 g, 354 mmol) in H2SO4 (260 mL) at 0°C with stirring, and then stirring for 2 hours. TLC (petroleum ether: ethyl acetate = 5:1, Rf = 0.57) detection indicates complete conversion of the reactants. The reaction mixture is poured into ice water (3 kg), the pH is adjusted to 7 with aqueous NaOH solution, then extracting with ethyl acetate (1.5 Lx 2). The extracted organic phase is dried over Na2SO4, and concentrated to obtain a crude product. The crude product is purified by a silica gel column (the eluent is: petroleum ether: ethyl acetate = 20: 1-5: 1) to obtain Compound b(91.0 g, yield: 69%) as a yellow solid. 1H NMR:(ES6131-2-P1A, CDCl3, 400MHz)δ7.45-7.43 (d, J = 9.2 Hz, 1H), 6.82-6.79 (d, J = 12.0 Hz, 1H), 3.89 (s, 3H), 3.69 (br s, 2H).
References:
Shenzhen Forward Pharmaceuticals Co., Ltd.;TALLEY, J. John;YANG, Xuan;ZHU, Chenggang;XU, Liangliang EP3705478, 2020, A1 Location in patent:Paragraph 0057; 0059
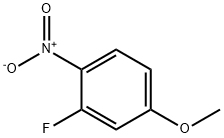
446-38-8
184 suppliers
$6.00/1g

1569986-91-9
30 suppliers
inquiry