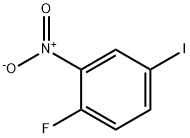
2-Fluoro-5-iodonitrobenzene synthesis
- Product Name:2-Fluoro-5-iodonitrobenzene
- CAS Number:364-75-0
- Molecular formula:C6H3FINO2
- Molecular Weight:267
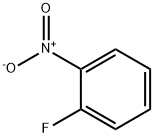
1493-27-2

364-75-0
The general procedure for the synthesis of 2-fluoro-5-iodonitrobenzene from 1-fluoro-2-nitrobenzene is as follows: Intermediate Example 3. Synthesis of 1-fluoro-4-iodo-2-nitrobenzene To a solution of trifluoromethanesulfonic acid (15.6 ml, 177.15 mmol, 5 eq.) of 1-fluoro-2-nitrobenzene (5 g, 35.43 mmol) was added N-iodosuccinimide (9.57 g, 42.5 mmol, 1.2 eq.) in one batch at 0 °C. The reaction mixture was stirred at room temperature for 1 hour. After completion of the reaction, the reaction was quenched by addition of water and extracted with diethyl ether (3 x 150 ml). The organic layers were combined and washed sequentially with water, aqueous sodium thiosulfate and brine and then dried over anhydrous sodium sulfate. The solvent was removed by distillation under reduced pressure and the crude product obtained was purified by column chromatography (60-120 mesh silica gel, 5% ethyl acetate in hexane solution as eluent) to afford the target compound 1-fluoro-4-iodo-2-nitrobenzene in 66% yield (6.2 g). 1H NMR (300 MHz, DMSO-d6): δ 8.42 (dd, 1H), 8.18-8.13 (m, 1H), 7.46-7.39 (m, 1H).
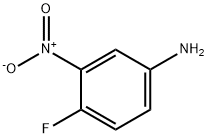
364-76-1
284 suppliers
$7.00/10g

364-75-0
97 suppliers
$5.00/250mg
Yield: 14%
Reaction Conditions:
with diiodomethane;isopentyl nitrite at 20 - 70; for 2 h;
Steps:
46
Add isoamyl nitrite (18. 6 g, 160 mmol) to a suspension of 4-fluoro-3- nitrophenylamine (5. 0 g, 32 mmol) in diiodomethane (150 mL). Stir under nitrogen at room temperature for 1 h. Heat at 70 °C for 1 h. Cool to room temperature and concentrate. Dilute with dichloromethane (500 mL) and water (100 mL). Collect the organic, concentrate and purify (silica gel chromatography, eluting with a gradient of 100 : 0 to 80 : 20 hexanes : ethyl acetate), to give the title compound as a yellow oil (1. 22 g, 14%). 1H NMR (300 MHz, CDC13) 6 7. 02-7. 11 (m, 1H), 7. 89-7. 97 (m, 1H), 8. 32-8. 39 (dd, J = 2. 2 Hz, 6. 9 Hz, 1H).
References:
ELI LILLY AND COMPANY WO2005/94822, 2005, A1 Location in patent:Page/Page column 46

1493-27-2
511 suppliers
$5.00/10g

364-75-0
97 suppliers
$5.00/250mg
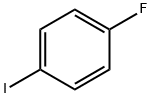
352-34-1
380 suppliers
$7.00/5g

364-75-0
97 suppliers
$5.00/250mg

364-76-1
284 suppliers
$7.00/10g
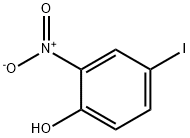
21784-73-6
53 suppliers
$75.00/500mg

364-75-0
97 suppliers
$5.00/250mg