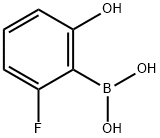
2-Fluoro-6-hydroxyphenylboronic acid synthesis
- Product Name:2-Fluoro-6-hydroxyphenylboronic acid
- CAS Number:1256345-60-4
- Molecular formula:C6H6BFO3
- Molecular Weight:155.92
2-Fluoro-6-hydroxyphenylboronic Acid is a synthetic reagent used in the preparation of alkylene-bridged [(3,4-dihydro-2H-benzo[b][1,4]oxazinyl) as HIV integrase inhibitors for treating HIV infection.It is synthesised from 2-Fluoro-6-methoxyphenylboronic acid by demethylation,reaction with boron tribromide in dichloromethane.
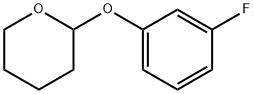
185913-28-4
3 suppliers
inquiry

1256345-60-4
149 suppliers
$7.00/100mg
Yield:1256345-60-4 50%
Reaction Conditions:
Stage #1: 2-(3-fluorophenoxy)tetrahydro-2H-pyranwith n-butyllithium in tetrahydrofuran at -80 - -70; for 1.5 h;Inert atmosphere;
Stage #2: with Trimethyl borate in tetrahydrofuran; for 2 h;
Steps:
1.2; 2.2 Step 2 Boronation Reaction
2-(3-fluorophenoxy)tetrahydropyran (313g, 1.595mol) and tetrahydrofuran 1600g are mixed and dissolved in a reaction bottle,Under nitrogen protection, the temperature was lowered to -70, 2.5M n-butyl lithium (702ml, 1.755mol) was added dropwise, and the temperature was kept at -70--80.After the dripping, the reaction took 1.5 hours.Trimethyl borate (199 g, 1.914 mol) was added dropwise, and the reaction was incubated for 2 hours, then naturally warmed to room temperature, and stirred overnight.The temperature of the reaction solution was lowered to -15°C, dripped in 900 ml of 3N hydrochloric acid, and then the ice bath was removedStir at room temperature, controlled by TLC, stir for 2 hours, the protection of tetrahydropyran is basically completed.The layers were separated, the aqueous layer was extracted twice with ethyl acetate, and the oil layers were combined,Wash once with saturated brine and dry with anhydrous magnesium sulfate,Rotary evaporate with suction until no liquid drops, add solvent heptane to beat, suction filterHeptane was rinsed to obtain a white solid. After drying, it was dried in vacuo to obtain 125 g of 2-fluoro-6-hydroxyphenylboronic acid. The HPLC measurement was 97%, and the yield was 50%
References:
CN111217843,2020,A Location in patent:Paragraph 0008; 0018; 0020; 0022
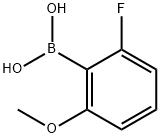
78495-63-3
251 suppliers
$9.00/1g

1256345-60-4
149 suppliers
$7.00/100mg
456-49-5
375 suppliers
$8.00/10g

1256345-60-4
149 suppliers
$7.00/100mg
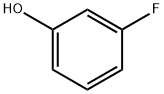
372-20-3
392 suppliers
$7.00/5g

1256345-60-4
149 suppliers
$7.00/100mg
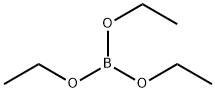
150-46-9
200 suppliers
$14.00/25mL

456-49-5
375 suppliers
$8.00/10g

1256345-60-4
149 suppliers
$7.00/100mg