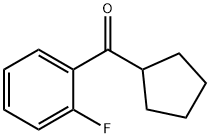
2-FLUOROPHENYL CYCLOPENTYL KETONE synthesis
- Product Name:2-FLUOROPHENYL CYCLOPENTYL KETONE
- CAS Number:111982-45-7
- Molecular formula:C12H13FO
- Molecular Weight:192.23
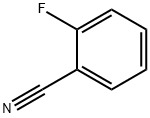
394-47-8
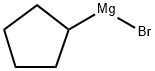
33240-34-5

111982-45-7
General procedure for the synthesis of 2-fluorophenylcyclopentanone from 2-fluorobenzonitrile and cyclopentylmagnesium bromide: 2-fluorobenzonitrile (5.0 g, 41.01 mmol) was added to 80 mL of THF containing 2 moles of cyclopentylmagnesium bromide in THF solution (20.51 mL, 41.01 mmol) and CuBr (0.100 g) under argon protection at 60 °C and the reaction was The reaction was stirred for 15 hours. After completion of the reaction, the reaction mixture was cooled to 0 °C, 5% sulfuric acid solution was added, and stirring was continued for 15 hours. Subsequently, the reaction mixture was extracted with ether three times. The organic layers were combined, dried with anhydrous MgSO4, concentrated and the residue was purified by column chromatography (eluent: a mixture of ethyl acetate and hexane) to afford the target product 2-fluorophenyl cyclopentanone (3.085 g, 40% yield). The mass spectrum (ES) showed m/z=192.15 (M+1)+.

394-47-8
479 suppliers
$5.00/5g

33240-34-5
187 suppliers
$88.00/100g

111982-45-7
54 suppliers
$35.00/1mg
Yield:111982-45-7 40%
Reaction Conditions:
Stage #1:2-fluorobenzonitrile;cyclopentylmagnesium bromide with copper(I) bromide in tetrahydrofuran at 60; for 15 h;
Stage #2: with sulfuric acid;water in tetrahydrofuran at 0; for 15 h;
Steps:
100
Preparation 100; Cvclopentvl- (2-fluoro-phenyl)-methanone Stir 2-Fluorobenzonitrile (5. 0g, 41.01 mmole) in 80 ml of THF with a 2 molar cylcopentyl magnesium bromide THF solution (20.51 ml, 41.01 mmole) and CuBr (0. 100g, 0.697 mmole) for 15 hrs at 60 °C under argon gas. Add al5% solution of sulfuric acid to the reaction at 0 °C and stir for 15 hrs. Extract the reaction mixture three times with diethyl ether. Combine organic layers and dry over MgS04 and concentrate Purify residue via column chromatography using mixture of Ethyl Acetate and hexanes ; to give 3.085 grams. Yield 40% MS (ES) = 192.15 (M+1) +.
References:
ELI LILLY AND COMPANY WO2005/66126, 2005, A1 Location in patent:Page/Page column 83
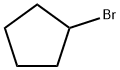
137-43-9
322 suppliers
$13.00/25g

394-47-8
479 suppliers
$5.00/5g

111982-45-7
54 suppliers
$35.00/1mg