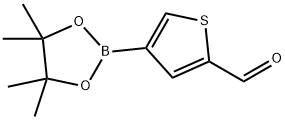
2-Formylthiophene-4-boronic acid pinacol ester synthesis
- Product Name:2-Formylthiophene-4-boronic acid pinacol ester
- CAS Number:881381-12-0
- Molecular formula:C11H15BO3S
- Molecular Weight:238.11
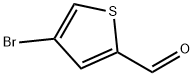
18791-75-8

73183-34-3

881381-12-0
General procedure for the synthesis of 2-formylthiophene-4-boronic acid pinacol ester from 4-bromo-2-thiophenecarboxaldehyde and bis(pinacolato)diboron (865 mg, 3.403 mmol), potassium acetate (667 mg, 6.81 mmol) and PdCl2(dppf) (96 mg, 0.131 mmol). The reaction mixture was placed in a microwave reactor and heated at 150 °C for 20 min. After completion of the reaction, all solvents were removed by evaporation. The residue was partitioned between ethyl acetate (100 mL) and water (100 mL). The aqueous layer was extracted with ethyl acetate (2 x 100 mL). The combined organic phases were washed with brine (100 mL), dried over anhydrous MgSO4 and concentrated. The crude product was purified by Combiflash fast column (elution gradient: hexane/ethyl acetate, 1%-30% ethyl acetate, 20 min) to afford pinacol ester of 2-formylthiophene-4-boronic acid (550 mg, 88% yield).LC/MS analysis: m/z 238.2 ([M+H]+), retention time 1.88 min.

18791-75-8
287 suppliers
$9.00/5g

73183-34-3
586 suppliers
$6.00/5g

881381-12-0
57 suppliers
$17.00/250mg
Yield:881381-12-0 88%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,2-dimethoxyethane at 150; for 0.333333 h;Microwave;
Steps:
42
To 4-bromo-2-thiophenecarbaldehyde (500 mg, 2.61 mmol) in DME (18 mL), bis(pinacolato)diboron (865 mg, 3.403 mmol), potassium acetate (667 mg, 6.81 mmol) and PdCI2(dppf) (96 mg, 0.131 mmol) were added. The reaction mixture was heated by microwave at 1500C for 20 minutes. Then all the solvent was evaporated. The residue was partitioned between ethyl acetate (100 mL) and water (100 mL). The water layer was extracted with ethyl acetate (2x100 mL). The combined organic phase was washed with brine (100 mL) and dried with Mg2SO4 and concentrated. The crude product was purified by Combiflash (Hexane/ethyl acetate, 1% -30 % ethyl acetate, 20 min.) to give title compound (550 mg, 88 %). LC/MS: m/z, 238.2 (M+H), 1.88 min
References:
WO2006/34317,2006,A2 Location in patent:Page/Page column 94