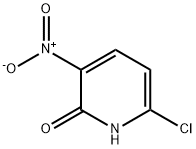
2-Hydroxy-3-Nitro-6-Chloropyridine synthesis
- Product Name:2-Hydroxy-3-Nitro-6-Chloropyridine
- CAS Number:92138-35-7
- Molecular formula:C5H3ClN2O3
- Molecular Weight:174.54
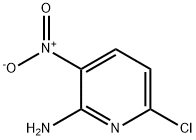
27048-04-0

92138-35-7
General procedure for the synthesis of 6-chloro-3-nitropyridin-2(1H)-one from 2-amino-3-nitro-6-chloropyridine: 6-chloro-3-nitropyridin-2-ylamine (Intermediate 4, 5 g, 28 mmol) was added to ice-cold concentrated sulfuric acid, and the reaction mixture was stirred for 10 min at 0 °C. Subsequently, NaNO2 solution (4 g, 58 mmol, dissolved in 30 mL of water) was added slowly over 10 min, keeping the reaction temperature at 0 °C and continued stirring at the same temperature for 30 min. Upon completion of the reaction, the mixture was poured into 150 mL of ice-cold water, the precipitate formed was collected by filtration, washed with cold water (2 x 50 mL), and dried under vacuum to afford 6-chloro-3-nitropyridin-2(1H)-one as a yellow solid in 80% yield (4 g, 23 mmol). Mass spectrum (ES): 172.5 (M-1) C5H3ClN2O3. 1H-NMR (DMSO-d6, 400MHz): δ 7.06 (d, J=8.3Hz, 1H), 8.40-8.43 (m, 1H).

27048-04-0
346 suppliers
$5.00/250mg

92138-35-7
92 suppliers
$10.00/250mg
Yield:92138-35-7 80%
Reaction Conditions:
Stage #1: 2-amino-6-chloro-3-nitropyridinewith sulfuric acid at 0; for 0.166667 h;
Stage #2: with sodium nitrite in water at 0; for 0.666667 h;
Steps:
5
Intermediate 5 6-Chloro-3-nitropyridin-2(l//)-one To ice cold concentrated sulphuric acid was added 6-chloro-3 -nitro-pyridin-2-ylamine (Intermediate 4, 5 g, 28 mmol), and the reaction mixture was stirred for 10 min at 0 0C. A solution OfNaNO2 (4 g, 58 mmol in 30 mL of water) was added slowly over a period of 10 min maintaining the temperature at 00C, then the mixture was stirred for 30 min at the same temperature. The reaction mixture was poured onto ice cold water (150 mL), the precipitate that formed was collected by filtration, washed with cold water (2x50 mL) and dried under vacuum to provide 6-chloro-3-nitropyridin-2(lf/)-one in 80% yield (4 g, 23 mmol) as yellow color solid. MS (ES): 172.9 (M-I) for C5H3ClN2O3.1H-NMR (DMSO-d6, 400 MHz): δ 7.06 (d, J= 8.3 Hz, IH), 8.40-8.43 (m, IH).
References:
WO2009/27732,2009,A1 Location in patent:Page/Page column 70
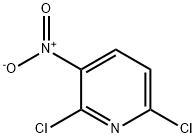
16013-85-7
498 suppliers
$9.00/5g

92138-35-7
92 suppliers
$10.00/250mg