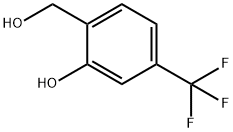
2-HydroxyMethyl-5-trifluoroMethyl-phenol synthesis
- Product Name:2-HydroxyMethyl-5-trifluoroMethyl-phenol
- CAS Number:349-66-6
- Molecular formula:C8H7F3O2
- Molecular Weight:192.14
328-90-5
331 suppliers
$10.00/25g

349-66-6
40 suppliers
$45.00/50mg
Yield: 93%
Reaction Conditions:
with borane-THF in tetrahydrofuran at 0 - 20; for 18 h;
Steps:
S14 Preparation of [2-(3,4-dichloro-phenyl)-ethyl]-(6-trifluoromethyl-benzofuran-2-ylmethyl)-amine
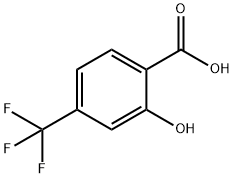
To a solution of 600 mg (2.91 mmol) of 2-hydroxy-4-trifluoromethyl-benzoic acid in THF (3 ml) were added dropwise 5.82 ml of a 1 molar solution of BH3-THF complex in THF at 0° C. After the reaction mixture was stirred for 18 h at RT, water was added to destroy excess reducing agent. Then 15 ml of 2N NaOH-solution were added and the mixture was stirred for 30 min. Then ether (10 ml) was added and the aqueous phase was separated. The volatile solvents of the organic phase were removed and the remaining residue was combined with the aqueous phase. The pH of the aqueous phase was then carefully adjusted to 6-7 by addition of dilute acetic acid at 0° C. and the mixture was extracted with ether. The combined extracts were then dried (MgSO4) and concentrated to yield 649 mg (93%) of crude 2-hydroxymethyl-5-trifluoromethyl-phenol (purity 80%) as a light yellow oil. MS (ISP) 191.2 (M-H)-.To a solution of 649 mg of 2-hydroxymethyl-5-trifluoromethyl-phenol (obtained in step a) were added 2 g of manganese dioxide and the mixture was stirred at RT for 16 h. After filtration, the filtrate was concentrated and purified by column chromatography (silica gel; cyclohexane/ethyl acetate 4:1). 2-Hydroxy-4-trifluoromethyl-benzaldehyde was isolated as a light yellow solid (292 mg, 57%). MS (ISP) 189.2 (M-H)-.In analogy to example S6 (steps a to c) 220 mg (1.16 mmol) 2-hydroxy-4-trifluoromethyl-benzaldehyde were converted into 275 mg of [2-(3,4-dichloro-phenyl)-ethyl]-(6-trifluoromethyl-benzofuran-2-ylmethyl)-amine. The compound was obtained as a dark brown oil. MS (ISP) 388.2 (M+H)+.
References:
Faeh, Christoph;Kuehne, Holger;Luebbers, Thomas;Mattei, Patrizio;Maugeais, Cyrille;Pflieger, Philippe US2007/185113, 2007, A1 Location in patent:Page/Page column 13