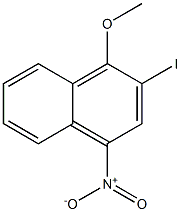
2-iodo-1-methoxy-4-nitronaphthalene synthesis
- Product Name:2-iodo-1-methoxy-4-nitronaphthalene
- CAS Number:1429765-70-7
- Molecular formula:C11H8INO3
- Molecular Weight:329.0906
4900-63-4
69 suppliers
$70.00/500mg

1429765-70-7
5 suppliers
inquiry
Yield:1429765-70-7 70%
Reaction Conditions:
with N-iodo-succinimide;trifluoroacetic acid for 20 h;Reflux;Inert atmosphere;Temperature;Time;
Steps:
2-Iodo-l-methoxy-4-nitronaphthalene (38)
2-Iodo-l-methoxy-4-nitronaphthalene (38). A mixture of commercially available 1-methoxy-4-nitronaphthalene (2.1 g, 10.4 mmol), N-iodosuccinimide (2.7 g, 12 mmol) in TFA (40 mL) was heated to reflux and stirred for 20 h under a N2 atmosphere. The reaction mixture was diluted with EtOAc (40 mL), washed with saturated aqueous Na2S203 solution (30 mL), saturated aqueous NaHCC>3 (30 mL x 2), and brine (30 mL). The organic layer was dried (MgS04), filtered and silica was added to filtrate and the solvent was removed under reduced pressure. The adsorbed crude residue was purified by flash column chromatography (100% hexane) on silica gel to give 38 (2.4 g, 70%) as a light yellow solid.]H NMR (400 MHz, CDC13) δ 8.59 (s, 1H), 8.58 (s, 1H), 8.21 (d, / = 8.42 Hz, 1H), 7.74 (t, 7 = 7.53 Hz, 1H), 7.65 (t, 7 = 7.53 Hz, 1H), 4.03 (s, 3H); 13C NMR (100 MHz, CDC13) δ 161.69, 142.85, 134.07, 129.99, 128.55, 128.08, 126.55, 123.92, 123.02, 83.35, 62.20; ESI MS: m/z 330.0 (M+H)+. Note: Rf of starting material and product are very close and a good separation is achieved with a relatively long silica gel column and 100% hexane gradient. >95% pure product is needed for pd-catalyzed coupling step.
References:
WO2013/52943,2013,A2 Location in patent:Page/Page column 109; 110