
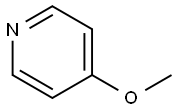
2-iodo-4-methoxypyridine synthesis
- Product Name:2-iodo-4-methoxypyridine
- CAS Number:89640-54-0
- Molecular formula:C6H6INO
- Molecular Weight:235.02
620-08-6
314 suppliers
$5.00/1g

89640-54-0
6 suppliers
inquiry
Yield:89640-54-0 39%
Reaction Conditions:
Stage #1: 4-methoxypyridinewith 2,2,6,6-tetramethylpiperidinylmagnesium chloride lithium chloride complex;boron trifluoride diethyl etherate in tetrahydrofuran;toluene at -20; for 20 h;
Stage #2: with iodine in tetrahydrofuran;toluene at 20;
Steps:
2-iodo-4 methoxypyridine (8) [30]
A solution of 4-methoxypyridine (2 g, 18.3 mmol) in 90 mL of dry THF containing BF3Et2O(2.5 mL, 20 mmol) was treated with TMPMgClLiCl (27 mL, 27 mmol, 1M toluene) at 20 C for 20 h,and the resulting mixture was quenched by adding the solution of iodine (9.14 g, 36 mmol) in 37 mL ofTHF. Following the warming to room temperature, the resulting mixture was quenched by adding100 mL of sat. aqueous NH4Cl and NH3 (9 mL) and sat. aqueous Na2S2O3 solution (18 mL), followedby extraction with Et2O (4 100 mL). The combined organic layer was dried over MgSO4, and thefiltrate was concentrated at reduced pressure. The residue was purified on silica gel using heptane andEt2O (4:1) as eluent, and 8 was isolated as light brown oil (1.65 g, 39% yield).1H-NMR (500MHz, DMSO): 8.13 (d, J = 5.8 Hz, 1H), 7.42 (d, J = 2.4 Hz, 1H), 7.01 (dd, J = 5.8 Hz,J = 2.4 Hz, 1H), 3.82 (s, 3H). 13C-NMR (124 MHz, DMSO): 166.0, 151.9, 120.3, 119.8, 111.4, 56.3. HR-MS:m/z calculated for C6H7INO ([M+H+]): 235.9567, found: 235.9571.
References:
Farkas, Emese;Gyorfi, Nandor;Kotschy, Andras;Nemet, Norbert;Novak, Zoltan;Weber, Csaba [Molecules,2020,vol. 25,# 20,art. no. 4766]