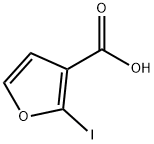
2-iodofuran-3-carboxylic acid synthesis
- Product Name:2-iodofuran-3-carboxylic acid
- CAS Number:152941-57-6
- Molecular formula:C5H3IO3
- Molecular Weight:237.98
488-93-7
377 suppliers
$5.00/250mg

152941-57-6
7 suppliers
inquiry
Yield:152941-57-6 62%
Reaction Conditions:
Stage #1: 3-Furoic acidwith n-butyllithium in tetrahydrofuran;hexane at -78 - 20; for 0.5 h;
Stage #2: with iodine in tetrahydrofuran;hexane at 20; for 2 h;
Steps:
2-Iodofuran-3-carboxylic acid
A solution of furan-3-carboxylic acid (4.41 g, 39.3 mmol) in THF (40 mL) was dropwise added to asolution of nBuLi (55.0 mL, 9.07 mol, 1.65 M in hexane) in THF at -78 oC. The mixture was allowed towarm to rt and stirred for 30 min. After cooling to -78 oC, a solution of I2 (11.0 g, 43.3 mmol) in THF (60mL) was dropwise added to the mixture, then the mixture was warmed to rt and allowed to stand for 2 h.After a 10% HCl aqueous solution was added to acidify the mixture, an extractive work-up with Et2O wascarried out. The organic layer was successively washed with Na2S2O3 and brine, then dried over MgSO4.After evaporation, the resulting residue was subjected to silica gel column chromatography withAcOH/1,1,2,2-tetrachloroethane (1/100) to give a colorless solid of the title compound (5.77 g, 62%), mp168.2-170.0 °C (CHCl3) [lit.10 mp 148-150 °C]. 1H-NMR (400 MHz, CDCl3) δ: 6.79 (1H, d, J = 2.0 Hz,4-H), 7.61 (1H, d, J = 2.0 Hz, 5-H). 13C-NMR (100 MHz, CDCl3) δ: 99.9, 112.9, 123.6, 148.7, 167.9. IR(KBr): νmax 741, 889, 1180, 1312, 1501, 1564, 1690 cm-1. Anal. Calcd for C5H3IO3: C, 25.23; H, 1.27. Found: C, 25.32; H, 1.45.
References:
Abe, Hitoshi;Kamimura, Mayu;Komatsu, Yoshinori;Horino, Yoshikazu [Heterocycles,2015,vol. 90,# 2,p. 1332 - 1342]