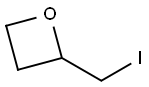
2-(IODOMETHYL)OXETANE synthesis
- Product Name:2-(IODOMETHYL)OXETANE
- CAS Number:121138-00-9
- Molecular formula:C4H7IO
- Molecular Weight:198
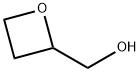
61266-70-4
132 suppliers
$75.40/250mg

121138-00-9
79 suppliers
$45.00/10mg
Yield: 100%
Reaction Conditions:
with 1H-imidazole;iodine;triphenylphosphine in dichloromethane at 0 - 25; for 18.5 h;
Steps:
1 Reference Example 1: Preparation of 2- (iodomethyl) oxetane
Triphenylphosphine (10.36 g, 39.5 mmol) and imidazole (5.4 g, 79.3 mmol) were dissolved in dichloromethane (70 ml) and cooled to 0 & lt; 0 & gt; C. To this mixture was slowly added iodine (10.36 g, 39.5 mmol) portionwise and stirred at the same temperature for 30 minutes. A solution of the above prepared 2-hydroxymethyloxetane (2.70 g, 30.68 mmol) dissolved in dichloromethane (30 ml) was added dropwise to the above solution, and the mixture was stirred at 0 ° C for 30 minutes and at 25 ° C for 18 hours The reaction was complete. The reaction solution was poured into ice water, the organic layer was separated, and the aqueous layer was extracted twice with dichloromethane (150 ml). The obtained organic layers were combined, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure.The obtained residue was purified by silica gel column chromatography (hexane: ethyl acetate = 6: 1) to obtain pure 2- (iodomethyl) oxetane (6.0 g, 100%).
References:
Korea Research Institute of Chemical Technology;Eseuti Pam Co., Ltd.;Kim Bong-jin;Kim Jae-hak;Lee Il-yeong;Lee Sang-ho;Lee Jong-gyo;Kim Gyeong-jin;Kim Uk-il;Nam Hwa-jeong KR101592370, 2016, B1 Location in patent:Paragraph 0085; 0086; 0088; 0089
115845-51-7
66 suppliers
$35.00/100mg

121138-00-9
79 suppliers
$45.00/10mg

627-27-0
411 suppliers
$8.00/1g

121138-00-9
79 suppliers
$45.00/10mg
121138-01-0
99 suppliers
$69.00/1g