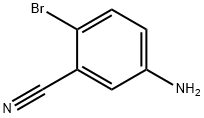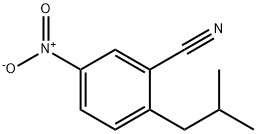
2-Isobutyl-5-nitrobenzonitrile synthesis
- Product Name:2-Isobutyl-5-nitrobenzonitrile
- CAS Number:288251-96-7
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23
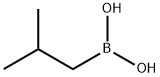
84110-40-7
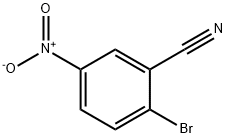
134604-07-2

288251-96-7
The general procedure for the synthesis of 2-isobutyl-5-nitrobenzonitrile from isobutylboronic acid and 2-bromo-5-nitrobenzonitrile was as follows: isobutylboronic acid (5.89 g), 2-bromo-5-nitrobenzonitrile (12.5 g), and Cs2CO3 (35.9 g) were dissolved in a solvent mixture of toluene (150 mL) and water (5 mL) under the protection of nitrogen and stirred at room temperature. Subsequently, PdCl2(dppf)-CH2Cl2 adduct (2.248 g) was added all at once. The reaction mixture was stirred at 100 °C for 16 hours. After completion of the reaction, it was cooled to room temperature and the solvent was removed by distillation under reduced pressure. The residue was purified by column chromatography to afford the target product 2-(2-methylpropyl)-5-nitrobenzonitrile (D42) (11 g) as a light yellow oil.1H NMR (CDCl3, 400 MHz) δ: 1.00 (6H, d), 2.06 (1H, m), 2.86 (2H, d), 7.52 (1H, d), 8.37 (1H, dd), 8.51 (1H, dd). dd), 8.51 (1H, d).
Yield:288251-96-7 56.3%
Reaction Conditions:
with caesium carbonate in water;toluene; for 2 h;Microwave irradiation;Heating;Inert atmosphere;
Steps:
31.1 Step 1:
Synthesis of 2-isobutyl-5-nitrobenzonitrile
To a mixture of 5-amino-2-bromobenzonitrile (1 g, 5.1 mmol), isobutylboronic acid (1.2 g, 11.8 mmol), caesium carbonate (2 g, 6.1 mmol), toluene (10 mL) and water (0.3 mL) in a 20 mL microwave tube was added Pd(dppf)Cl2 (350 mg, 0.48 mmol) under nitrogen atmosphere, and the mixture was heated to react at 120□ under microwave for 2 hours.
After completion of the reaction, solvent was removed in vacuo, then saturated sodium chloride (20 mL) was added.
The mixture was extracted with ethyl acetate (8 mL), dried over anhydrous sodium sulfate.
The organic layers were combined and concentrated in vacuo, and the crude product was separated by a silica gel column (petroleum ether: ethyl acetate = 10:1) to give a product (white solid, 0.5 g), with a yield of 56.3%. MS (ESI) m/z:175.1(M+1).
References:
EP3581561,2019,A1 Location in patent:Paragraph 0179; 0171

84110-40-7
338 suppliers
$6.00/1g

134604-07-2
118 suppliers
$9.00/1g

288251-96-7
25 suppliers
inquiry