
2-Methoxy-4-(1-methyl-4-piperidinyl)aniline synthesis
- Product Name:2-Methoxy-4-(1-methyl-4-piperidinyl)aniline
- CAS Number:1124330-14-8
- Molecular formula:C13H20N2O
- Molecular Weight:220.31

1625678-62-7
1 suppliers
inquiry

1124330-14-8
11 suppliers
inquiry
Yield:1124330-14-8 99%
Reaction Conditions:
with hydrogen;palladium(II) hydroxide in ethanol at 20; under 2585.81 Torr; for 4 h;
Steps:
19 Preparation of 2-methoxy-4-(i -methylpiperidin-4-yl)aniline (1 9b)
Preparation of 2-methoxy-4-(i -methylpiperidin-4- yl)aniline (1 9b): A 25 mE glass microwave tube was charged with potassium phosphate tribasic (3.00 g, 13.05 mmol), 2-(dicyclohexylphosphino)-2’,4’,6’,-tri-isopropyl- 1,1 ‘-biphenyl (Strem Chemicals, Newburyport, Mass., 83 mg, 0.174 mmol), tris(dibenzylideneacetone) dipalladium (0) (Strem Chemicals, Newburyport, Mass., 80mg, 0.O87mmol), 1-methyl- 1 ,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester (Acros Organics, New Jersey, 971 mg, 4.35 mmol) followed by 5-chloro-2-nitroanisole (Sigma Aldrich, 816 mg, 4.35 mmol). The solids were purged with argon and treated with 1 ,4-dioxane (12 mE) and water (4 mE), scaled and heated at1100 C. in a heating block for 1 h. The reaction mixture was treated with 1 N NaOH and extracted with EtOAc (3x30 mE), dried over Mg504, filtered and concentrated. The crude resi41due was purified on the ISCO Combiflash RF (80 g Thomson SingleStep column, using a gradient of 0-20% MeOH in DCM) affording 4-(3-methoxy-4-nitrophenyl)- 1-methyl-i ,2, 3,6-tetrahydropyridine (1 9a; 970 mg, 3.91 mmol, 90% yield) as a rust-brown solid which crystallized upon standing. mlz (ESI, +ve ion) 249.1 (M+H). ‘H NMR (400 MHz, CDC13) ? ppm 7.86 (1H, d, J=8.4 Hz), 6.99-7.08 (2H, m), 6.17-6.24 (1H, m), 3.97 (3H, s), 3.15 (2H, q, J=2.8 Hz), 2.65-2.74 (2H, m), 2.53-2.62 (2H, m), 2.38-2.46 (3H, m). In a 50 mE glass reactor, 4-(3-methoxy-4-nitrophenyl)- i-methyl-i ,2,3,6-tet- rahydropyridine (940 mg, 3.79 mmol) was treated with palladium hydroxide (20 wt % Pd, dry basis, on wet carbon, degussa type elOl ne/w, 266 mg, 0.38 mmol) and anhydrous EtOH (20 mE). The reactor was purged with hydrogen (5x) and allowed to stir under 50 psi hydrogen at RT for 4 h. The reaction mixture was filtered through a 0.45 urn acrodisc to remove the catalyst residues washing with MeOH and concentrated to dryness under high vacuum affording 2-meth- oxy-4-(i-methylpiperidin-4-yl)aniline (19b; 830 mg, 3.77 mmol, 99% yield) as a yellow crystalline solid. mlz (ESI, +ve ion) 221.0 (M+H). ‘H NMR (400 MHz, CDC13) ? ppm 6.56-6.74 (3H, m), 3.76-3.89 (3H, m), 3.54-3.76 (2H, m),2.97 (2H, d, J=ii.2 Hz), 2.34-2.47 (1H, m), 2.23-2.34 (3H,1.95-2.12 (2H, m), 1.70-1.90 (4H, m).
References:
US2014/249131,2014,A1 Location in patent:Paragraph 0265; 0266
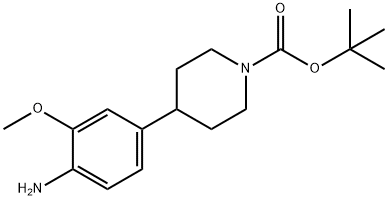
1089280-53-4
27 suppliers
inquiry

1124330-14-8
11 suppliers
inquiry
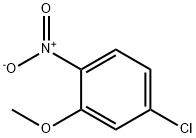
6627-53-8
165 suppliers
$10.00/1g

1124330-14-8
11 suppliers
inquiry

1233145-65-7
4 suppliers
inquiry

1124330-14-8
11 suppliers
inquiry
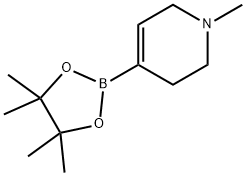
454482-11-2
152 suppliers
$9.00/250mg

1124330-14-8
11 suppliers
inquiry