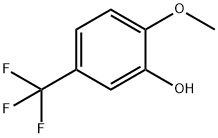
2-METHOXY-5-(TRIFLUOROMETHYL)PHENOL synthesis
- Product Name:2-METHOXY-5-(TRIFLUOROMETHYL)PHENOL
- CAS Number:349-67-7
- Molecular formula:C8H7F3O2
- Molecular Weight:192.14
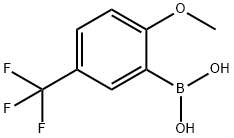
240139-82-6

349-67-7
The general procedure for the synthesis of 2-methoxy-5-trifluoromethylphenol from 2-methoxy-5-trifluoromethylphenylboronic acid was as follows: to a 100 mL round bottom flask was added [2-methoxy-5-(trifluoromethyl)phenyl]boronic acid (300 mg, 1.36 mmol, 1.00 eq.), ethanol (15 mL), and 30% hydrogen peroxide aqueous solution (2 mL). The reaction mixture was stirred at 80°C for 2 hours. Upon completion of the reaction, the mixture was concentrated under reduced pressure. Subsequently, water was added to dilute the reaction mixture. The mixture was washed with aqueous sodium sulfite and the aqueous layer was extracted with ethyl acetate and the organic layers were combined. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate and finally concentrated under reduced pressure to give 250 mg (95% yield) of 2-methoxy-5-(trifluoromethyl)phenol as a colorless oil.

240139-82-6
98 suppliers
$24.00/1g

349-67-7
41 suppliers
$45.00/50mg
Yield:349-67-7 95%
Reaction Conditions:
with dihydrogen peroxide in ethanol at 80; for 2 h;
Steps:
27.1 Synthesis of 2-methoxy-5-(trifluoromethyl)phenol
Into a 100-mL round-bottom flask, were placed [2-methoxy-5-(trifluoromethyl) phenyl] boronic acid (300 mg, 1 .36mmol, 1 .00 equiv), ethanol (15m l_), H202 (aq 30%) (2m l_).The resulting reaction was stirred for 2 h at 80°C. The resulting mixture was concentrated in vacuo. The reaction was then diluted by the addition of water. The resulting mixture was washed with Na2S03 (aq). The aqueous layer was extracted with ethyl acetate and the organic layers combined. The resulting mixture was washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuo. This resulted in 250 mg (95%) of 2-methoxy-5-(trifluoromethyl)phenol as a colorless oil.
References:
WO2015/130957,2015,A1 Location in patent:Page/Page column 85
402-52-8
136 suppliers
$14.00/1g

349-67-7
41 suppliers
$45.00/50mg