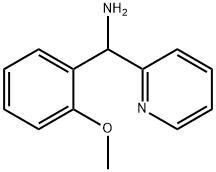
(2-methoxyphenyl)-(2-pyridyl)methanamine synthesis
- Product Name:(2-methoxyphenyl)-(2-pyridyl)methanamine
- CAS Number:95899-01-7
- Molecular formula:C13H14N2O
- Molecular Weight:214.26
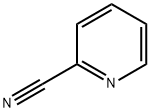
100-70-9
425 suppliers
$13.00/25g
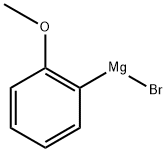
16750-63-3
64 suppliers
$66.09/100ml

95899-01-7
7 suppliers
inquiry
Yield:95899-01-7 95%
Reaction Conditions:
Stage #1: 2-Cyanopyridine;2-methoxyphenylmagnesium bromide in tetrahydrofuran;toluene at 0 - 5; for 1 h;
Stage #2: with 2-methyl-propan-1-ol in toluene at 15;
Stage #3: with sodium tetrahydroborate;watermore than 3 stages;
Steps:
1.1
EXAMPLE 1 (General Procedure A) 2- [1- (2-Methoxy-phenyl)-imidazo [1, 5] pyridin-3-yl, -pyrrolidin2-1- carboxylic acid (1-phenyt ethyl)-amide. S isomer Step 1; C- (2-methoxy-phenyl)-C-pyridin-2-yl-methylamine; 2-Cyanopyridine (7g, 0. 068mol) was dissolved in anhydrous toluene (225mL) and cooled to 0-5°C and 2-methoxyphenylmagnesium bromide (1. OM sol in THF, 75mmol, 75mL) was added dropwise over 30 minutes. The suspension was stirred for a further 30 minutes at this temperature then iso-butanol (90mL) was added keeping the temperature below 15°C. The suspension was cooled to 0°C and sodium borohydride (3.6g, 95mmol) added portion wise and the solution was stirred for a further 18 hours. The reaction was quenched with aqueous methanol, evaporated, extracted with dichloromethane and the organic layer concentrated under reduced pressure to afford a green oil (13. 8g, 95%) as the crude compound. HPLC 88%, Rt = 1.44 min. MS (AP) m/z 215 (M++H).
References:
WO2005/80390,2005,A1 Location in patent:Page/Page column 13-14