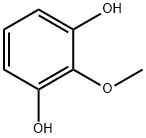
2-METHOXYRESORCINOL synthesis
- Product Name:2-METHOXYRESORCINOL
- CAS Number:29267-67-2
- Molecular formula:C7H8O3
- Molecular Weight:140.14
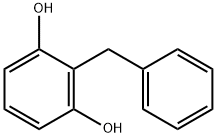
3769-40-2
2 suppliers
inquiry

29267-67-2
51 suppliers
$499.19/250mg
Yield: 68%
Reaction Conditions:
with lithium hydroxide in tetrahydrofuran;methanol;dichloromethane;water
Steps:
20 Novobiocin Analogs with Mono- or Dihydroxylated Furanose or Pyranose Noviose Replacements
To a solution of 14 (54 mg, 0.106 mmol) in THF: MeOH: H2O (1.5:1:1 mL) was added lithium hydroxide (27 mg, 0.53 mmol) at room temperature. The reaction mixture was stirred for 1 hour and neutralized with saturated ammonium chloride solution, extracted with ethyl acetate (3*5 mL), washed with brine. The combined organic layers were dried over anhydrous Na2SO4, and concentrated under vacuum. The residue was purified by column chromatography on silica using dichloromethane and methanol (97:3) as eluent to give 19 (35 mg, 68%) as a white amorphous solid. 1H NMR (500 MHz, acetone-d6) δ 9.14 (br s, 1H), 8.76 (s, 1H), 8.71 (s, 1H), 7.77 (d, 1H, J=1.9 Hz), 7.71 (dd, 1H, J=1.9, 8.4 Hz), 7.50 (d, 1H, J=8.6 Hz), 7.17 (d, 1H, J=8.7 Hz), 6.99 (d, 1H, J=8.4 Hz), 5.67 (d, 1H, J=1.7 Hz), 5.38 (m, 1H), 4.53 (m, 1H), 4.39 (m, 1H), 4.17 (dd, 1H, J=5.3, 9.3 Hz), 3.89 (dd, 1H, J=3.8, 9.3 Hz), 3.40 (d, 1H, J=7.3 Hz), 2.26 (s, 3H), 1.75 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 166.0, 159.5, 157.2, 149.9, 133.4, 129.9, 126.5, 126.0, 124.1, 124.0, 123.3, 122.9, 115.7, 115.2, 15.1, 113.0, 107.9, 79.2, 77.2, 73.9, 71.2, 28.8, 25.9, 17.9, 8.4. IR (KBr) νmax 3396, 2962, 2925, 1701, 1604, 1504, 1369, 1253, 1054, 981 cm-1. HRMS (ESI) m/z. [M-H-] calcd for C26H26NO8 480.1658. Found 480.1658.
References:
UNIVERSITY OF KANSAS US2011/82098, 2011, A1

133932-61-3
21 suppliers
$60.00/10 mg

29267-67-2
51 suppliers
$499.19/250mg
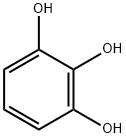
87-66-1
687 suppliers
$6.00/10g

74-88-4
357 suppliers
$15.00/10g

29267-67-2
51 suppliers
$499.19/250mg

74-88-4
357 suppliers
$15.00/10g

29267-67-2
51 suppliers
$499.19/250mg

87-66-1
687 suppliers
$6.00/10g

29267-67-2
51 suppliers
$499.19/250mg