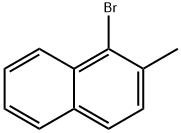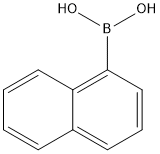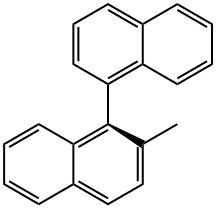
2-Methyl-1,1'-binaphthalene synthesis
- Product Name:2-Methyl-1,1'-binaphthalene
- CAS Number:118018-45-4
- Molecular formula:C21H16
- Molecular Weight:268.35
90-14-2
294 suppliers
$19.00/5g
103989-84-0
67 suppliers
$60.00/250mg

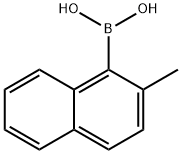
118018-45-4
6 suppliers
inquiry
Yield:118018-45-4 100%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);potassium carbonate in tetrahydrofuran at 20; for 2 h;Inert atmosphere;Suzuki coupling;
Steps:
6. General procedure for coupling reactions of aryl halides with organoboronic acids
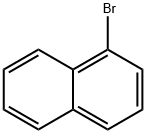
General procedure: A typical procedure is given for the reaction represented by Entry 8 in Table 1. Ligand 2e (6 mg, 0.01 mmol), Pd2(dba)3 (5 mg, 0.005 mmol), 2-methylnaphthyl-1-boronic acid (223 mg, 1.2 mmol), Cs2CO3 (975 mg, 3 mmol) were introduced to a flask under N2 gas. 1-bromo-2-methylnaphthalene (221 mg, 1 mmol) was added into the flask, followed by addition of THF (5 ml) by a syringe. The mixture was stirred under reflux for 24 h, under ambient pressure of N2. The solvent was then removed under reduced pressure. The resultant residual mixture was diluted with H2O (10 ml) and Et2O (10 ml), followed by extraction twice with Et2O. The organic extract was collected and stripped of solvent under vacuum. The product was isolated by column chromatography on silica eluting with hexane/ethyl acetate to give 276 mg (98%) of 2,2'-dimethyl-1-1'-binaphthalene as a solid, which was verified by GC/MS.
References:
Teo, Shihui;Weng, Zhiqiang;Hor, T.S. Andy [Journal of Organometallic Chemistry,2011,vol. 696,# 17,p. 2928 - 2934] Location in patent:experimental part
90-11-9
541 suppliers
$5.00/10g

103989-84-0
67 suppliers
$60.00/250mg

118018-45-4
6 suppliers
inquiry