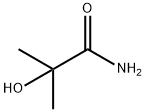
2-Methyl-2-hydroxypropionamide synthesis
- Product Name:2-Methyl-2-hydroxypropionamide
- CAS Number:13027-88-8
- Molecular formula:C4H9NO2
- Molecular Weight:103.12
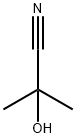
75-86-5

13027-88-8
The general procedure for the synthesis of 2-hydroxyisobutyramide from acetone cyanohydrin (ACH) is as follows: Hydration reaction Example 1 (0151) was carried out using the reaction apparatus shown in Figure 1. Reactors 1a, 1b and 1c were made of glass with an inner diameter of about 18 mm, equipped with jackets (as first, second and third reactors, respectively). The first reaction region 2a was filled with 16 g of pre-prepared catalyst, the second reaction region 2b was similarly filled with 16 g of catalyst and the third reaction region 2c was filled with 8 g of catalyst. The first reaction feedstock liquid is supplied at a rate of 14.8 g/hr through supply line 3a and is composed of 55.5 wt% pure water, 9.5 wt% acetone, and 35 wt% ACH. the second reaction feedstock liquid is supplied at a rate of 2.47 g/hr through supply line 3b and is composed of 17 wt% pure water, 13 wt% acetone, and 70 wt% ACH. the first reaction feedstock liquid of the ACH accounted for 75 wt% of the total ACH supply. The first oxidizer supply line 4a is supplied with oxygenated gas (9% oxygen and 91% nitrogen, v/v) at a rate of 26.7 ml/hr. This gas passes through the first reaction area 2a and enters the reaction liquid pool 12a via the effluent line 11a, and is subsequently completely discharged from the system via the withdrawal line 5a. A second oxidizer supply line 4b supplies, at the same rate, an oxygenated gas of the same composition, which passes through the second reaction region 2b, enters the reaction liquid pool 12b via the effluent line 11b, and is subsequently directed through the liquid supply line 10c to the third reactor 1c, which ultimately exits the system through the third reaction region 2c and the effluent line 11c. The outlet reaction liquid from the first reaction region is collected in the reaction liquid pool 12a and partially circulated to the inlet of the first reaction region through the circulation line 6a at a rate of 120 g/hr, with a circulation ratio of 8.1. The reaction liquid pool 12a is equipped with a level sensor, and the effluent in excess of 120 g/hr is transferred to the second reaction region through the transfer line 13a, with a constant level controlled by the sensor and the transfer pump 8t. The transfer rate is 14.8 g/hr. The outlet reaction liquid of the second reaction zone is collected in the reaction liquid pool 12b and partially circulated to the inlet of the second reaction zone through the circulation line 6b at a rate of 26.5 g/hr, with a circulation ratio of 1.5. The effluent in excess of 26.5 g/hr is transferred to the third reactor 1c through the liquid transfer line 13b. Note that the oxygenated gas supplied by the second oxidant supply line is transported to the third reactor.
Yield:-
Reaction Conditions:
with sulfuric acid in waterIndustry scale;
Steps:
The first stage of producing either MAA or methacrylate esters from ACH involves hydrolysis of the ACH in a hydrolysis system; the general chemistry of the hydrolysis process for both MAA and methacrylate esters may be very similar. Shown at 12 and 32 in FIG. 1 is hydrolysis of the ACH in a hydrolysis system to produce a hydrolysis mixture that contains α-sulfatoisobutyramide (“SIBAM”) and α-hydroxyisobutyramide (“HIBAM”). ACH and an excess of sulfuric acid are fed into hydrolysis reactor 12. It is preferable to use ACH that has low levels of water and other impurities in the hydrolysis step. The sulfuric acid serves as both a specific reactant and a solvent for the reaction. Using sulfuric acid at concentrations of greater than 95% is also preferred. In an alternative embodiment, oleum or a combination of sulfuric acid and oleum may be used in place of sulfuric acid. Either hydrolysis system may contain a single reactor or multiple reactors connected in series and may also employ one or more reactant addition points.
References:
Carlson JR., Curtis Ingstad;DeCourcy, Michael Stanley;Juliette, Jamie Jerrick John US2003/208093, 2003, A1 Location in patent:Page/Page column 3; Sheet 1
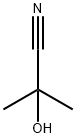
75-86-5
0 suppliers
$80.00/5g

13027-88-8
114 suppliers
$21.00/1g
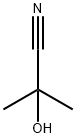
75-86-5
0 suppliers
$80.00/5g
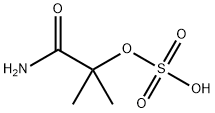
49562-37-0
1 suppliers
inquiry

13027-88-8
114 suppliers
$21.00/1g
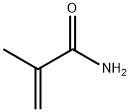
79-39-0
315 suppliers
$13.00/25g
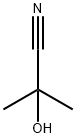
75-86-5
0 suppliers
$80.00/5g
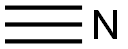
74-90-8
0 suppliers
inquiry

13027-88-8
114 suppliers
$21.00/1g

67-64-1
0 suppliers
$17.30/10ml