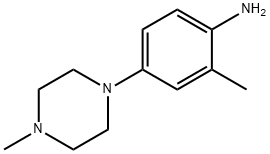
2-METHYL-4-(4-METHYLPIPERAZIN-1-YL)ANILINE synthesis
- Product Name:2-METHYL-4-(4-METHYLPIPERAZIN-1-YL)ANILINE
- CAS Number:16154-71-5
- Molecular formula:C12H19N3
- Molecular Weight:205.3
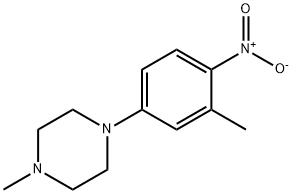
16154-61-3
35 suppliers
$31.80/250mgs:

16154-71-5
31 suppliers
$45.00/50mg
Yield:16154-71-5 800 mg
Reaction Conditions:
Stage #1: 1-methyl-4-(3-methyl-4-nitrophenyl)piperazinewith palladium 10% on activated carbon in ethanol at 20; for 0.25 h;Inert atmosphere;
Stage #2: with ammsnium formate in ethanol; for 0.0333333 h;Inert atmosphere;
Steps:
2 Step 2: 2-Methyl-4-(4-methylpiperazin- 1 -yl)aniline
To a solution of l-methyl-4-(3-methyl-4-nitrophenyl)piperazine (1.0 g, 4.25 mmol) in ethanol (20 mL) was added catalytic amount of 10% palladium on carbon and the mixture was stirred at RT for 15 min under nitrogen atmosphere. Ammonium formate (2.6 g, 42.5 mmol) was added to the mixture and stirred for 2 min. The mixture was cooled to RT and filtered through celite. The filtrate was concentrated, dissolved in ethyl acetate and the organic solution was washed with saturated sodium bicarbonate solution followed by brine and dried over anhydrous sodium sulfate. The solution was filtered and concentrated under reduced pressure to yield 800 mg of the desired product. NMR (400 MHz, DMSO-de) d 2.02 (s, 3H), 2.20 (s, 3H), 2.67-2.72 (m, 4H), 2.87-2.91 (m, 4H), 4.33 (br s, 2H), 6.48-6.56 (m, 2H), 6.59 (d, J = 2.4 Hz, 1H); ESI-MS ( m/z ) 206 (M+H)+.
References:
WO2020/70331,2020,A1 Location in patent:Page/Page column 70
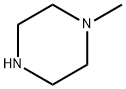
109-01-3
672 suppliers
$5.00/5G

16154-71-5
31 suppliers
$45.00/50mg
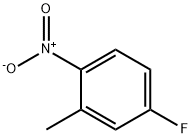
446-33-3
257 suppliers
$20.00/25g

16154-71-5
31 suppliers
$45.00/50mg

109-01-3
672 suppliers
$5.00/5G

446-33-3
257 suppliers
$20.00/25g

16154-71-5
31 suppliers
$45.00/50mg