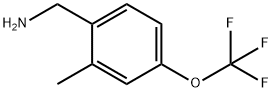
(2-Methyl-4-(trifluoroMethoxy)phenyl)MethanaMine synthesis
- Product Name:(2-Methyl-4-(trifluoroMethoxy)phenyl)MethanaMine
- CAS Number:771572-39-5
- Molecular formula:C9H10F3NO
- Molecular Weight:205.18

261951-90-0
17 suppliers
$343.00/1g

771572-39-5
15 suppliers
$124.00/250mg
Yield:771572-39-5 95%
Reaction Conditions:
Stage #1: 2-methyl-4-(trifluoromethoxy)benzamidewith borane-THF in tetrahydrofuran at 0; for 8 h;Heating / reflux;
Stage #2: with hydrogenchloride;water in tetrahydrofuran at 0; for 1 h;Heating / reflux;
Stage #3: with sodium hydroxide;water in tetrahydrofuran;
Steps:
5A
Example 5A2-Methyl-4-(trifluoromethoxy)benzylamine; 18.8 ml (18.8 mmol) of borane-THF complex (1M) are provided under argon with ice cooling. A solution of 823 mg (3.76 mmol) of 2-methyl-4-(trifluoromethoxy)benzamide (Example 4A) in 80 ml of THF is added dropwise and then the mixture is stirred under reflux for 8 h. With ice cooling, 80 ml of 1N hydrochloric acid are added dropwise (until the evolution of gas comes to an end) and the mixture is heated under reflux for 1 h. The reaction mixture is then rendered alkaline with a 1N sodium hydroxide solution and extracted three times with dichloromethane, the combined organic phases are dried over sodium sulfate and the solvent is removed under vacuum. This gives an oil which is reacted further without further purification. Yield: 732 mg (95% of theory).LC-MS (method 3): Rt=1.41 min.MS (ESI+): m/z=206 (M+H)+ 1H NMR (400 MHz, CDCl3): δ=7.32-7.40 (m, 1H), 6.99-7.11 (m, 2H), 3.95-4.01 (m, 2H), 2.40 (s, 3H).Adding excess HCl in dioxane (4N) and removing the volatile components on a rotary evaporator gives the corresponding hydrochloride.
References:
US2009/181996,2009,A1 Location in patent:Page/Page column 10

261951-91-1
30 suppliers
$123.70/1gm:

771572-39-5
15 suppliers
$124.00/250mg