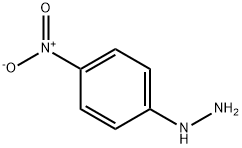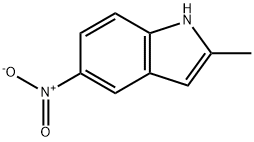
2-METHYL-5-NITROINDOLE synthesis
- Product Name:2-METHYL-5-NITROINDOLE
- CAS Number:7570-47-0
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
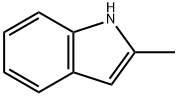
95-20-5
407 suppliers
$5.00/10g

7570-47-0
93 suppliers
$14.00/250mg
Yield:7570-47-0 96%
Reaction Conditions:
with sodium nitrate;sulfuric acid at 0; for 0.166667 h;
Steps:
Intermediate : 2-Methyl-5 -nitro -1/ - indole
To a vigorously stirred solution of commercially available 2- methyl-indole ( 262 mg, 2 mmol) in H2SO4 (2 mL) at 0 °C, A solution of NaNCb (187 mg, 2.2 mmol) in H2SO4 (2 mL) was added dropwise. Then, the reaction was stirred for another 10 minutes, and then poured into 8 mL of ice-water, precipitating a yellow product. The product was isolated via filtration and washed with cold water. 335 mg of yellow solid (96%). NMR (400 MHz) (DMSO) d) 11.68 (s, 1 H), 8.38 (s, 1 H), 7.90 (d, 1 H, J= 8.8 Hz), 7.40 (d, 1 H, J= 8.8 Hz), 6.37 (s, 1 H), 2.41 (s, 3 H). 13C NMR (100 MHz) (DMSO) 5140.48, 139.90, 139.42, 127.96, 115.81, 115.61, 110.63, 101.58, 13.32.
References:
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY;HU, Longqin;ABED, Dhulfiqar, Ali WO2020/150446, 2020, A1 Location in patent:Paragraph 0474-0475
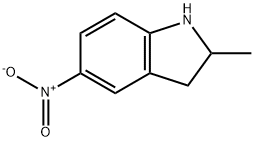
115210-54-3
24 suppliers
$45.00/100mg

7570-47-0
93 suppliers
$14.00/250mg