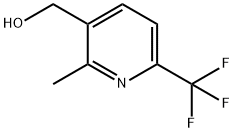
[2-Methyl-6-(trifluoromethyl)pyridin-3-yl]methanol synthesis
- Product Name:[2-Methyl-6-(trifluoromethyl)pyridin-3-yl]methanol
- CAS Number:681260-50-4
- Molecular formula:C8H8F3NO
- Molecular Weight:191.15
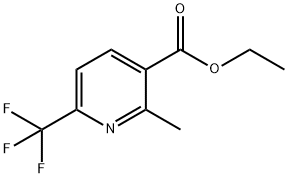
380355-65-7
68 suppliers
$60.00/100mg
![[2-Methyl-6-(trifluoromethyl)pyridin-3-yl]methanol](/CAS/GIF/681260-50-4.gif)
681260-50-4
36 suppliers
$45.00/50mg
Yield:681260-50-4 85%
Reaction Conditions:
Stage #1: ethyl 2-methyl-6-(trifluoromethyl)pyridine-3-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 20; for 3 h;
Stage #2: with sodium hydroxide;water in tetrahydrofuran;
Steps:
3.I34
3) Methods for making pyridine derivativesExample 134: Preparation of r2-methvl-6-trifluoromethvlpyridin-3-vH-methanolLiAIf-LEthyl 2-methyl-6-trifluoromethylnicotinate (4.66 g, 0.02 mol) (preparation according to Heterocycles 1997 (129) 46 and WO 01/0194339) in tetrahydrofuran was added slowly to a suspension of (1.9 g, 0.05 mol) of lithium aluminium hydride in tetrahydrofuran (150 ml) under nitrogen at room temperature. The reaction mixture was stirred at room temperature for 3 hours and was then quenched by sequential addition of water (0.9 ml), aqueous sodium hydroxide (0.9 ml) (3M), and water (2.7 ml). The sludge was filtered through celite. The residue was washed with diethyl ether and the washings combined with the filtrate. The combined liquors were concentrated under reduced pressure to give [2-methyl-6-trifluoromethylpyridin-3-yl]-methanol (3.92 g, 85% purity), which was used directly without further purification. 1H-NMR (400 MHz, CDCl3): 2.58 (s, 3H, Me), 4.81 (s, 2H, CH2), 7.57 (d, IH, CH), 7.94 (d, lH, CH) ppm.
References:
WO2007/71900,2007,A1 Location in patent:Page/Page column 93
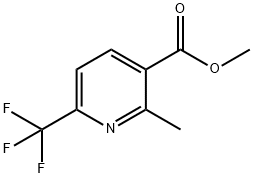
205582-88-3
41 suppliers
$26.00/250mg
![[2-Methyl-6-(trifluoromethyl)pyridin-3-yl]methanol](/CAS/GIF/681260-50-4.gif)
681260-50-4
36 suppliers
$45.00/50mg

205582-88-3
41 suppliers
$26.00/250mg
![[2-Methyl-6-(trifluoromethyl)pyridin-3-yl]methanol](/CAS/GIF/681260-50-4.gif)
681260-50-4
36 suppliers
$45.00/50mg
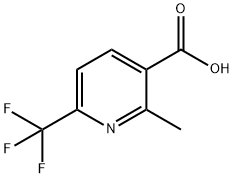
261635-93-2
100 suppliers
$22.00/250mg
![[2-Methyl-6-(trifluoromethyl)pyridin-3-yl]methanol](/CAS/GIF/681260-50-4.gif)
681260-50-4
36 suppliers
$45.00/50mg