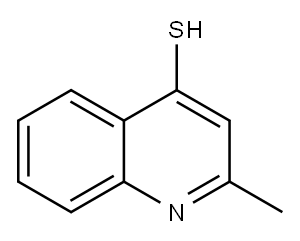
2-METHYL-QUINOLINE-4-THIOL synthesis
- Product Name:2-METHYL-QUINOLINE-4-THIOL
- CAS Number:90945-94-1
- Molecular formula:C10H9NS
- Molecular Weight:175.25
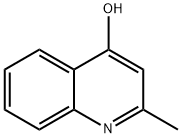
607-67-0
166 suppliers
$10.00/1g

90945-94-1
15 suppliers
inquiry
Yield:90945-94-1 30%
Reaction Conditions:
with Lawessons reagent in tetrahydrofuran at 80; for 6 h;
Steps:
5.5A
Step 5A Preparation of 2-methylquinoline-4-thiol 5a A stirred suspension of 2-methylquinolin-4-ol (5.10 g, 32.0 mmol, 1.0 eq.) in tetrahydrofuran (51 mL) was heated to 50° C., and 6.5 g of Lawesson's Reagent (6.5 g, 16.0 mmol, 0.5 eq.) was added in one portion. The reaction was heated to 80° C. and stirred at that temperature for 6 hours. The reaction was poured into a hot biphasic solution of ethyl acetate (200 mL) and water (200 mL) and was vigorously stirred. A viscous orange gel precipitated from the solution. The water/ethyl acetate solution was decanted into a separating funnel and the organic layer isolated. The aqueous layer was extracted with ethyl acetate (4×200 ml), and the combined extracts were dried over magnesium sulfate. The solution was filtered and concentrated in vacuo. The resulting orange gel was purified by flash chromatography (50% EtOAc:50% petroleum ether→100% EtOAc) to yield 5a as a yellow solid (1.70 g, 30%).
References:
US2008/300240,2008,A1 Location in patent:Page/Page column 15
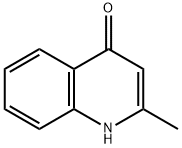
5660-24-2
49 suppliers
inquiry

90945-94-1
15 suppliers
inquiry