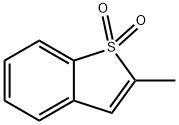
2-methylbenzothiophene 1,1-dioxide synthesis
- Product Name:2-methylbenzothiophene 1,1-dioxide
- CAS Number:6224-55-1
- Molecular formula:C9H8O2S
- Molecular Weight:180.22
![2-METHYLBENZO[B]THIOPHENE](/CAS/GIF/1195-14-8.gif)
1195-14-8
105 suppliers
$16.00/100mg

6224-55-1
5 suppliers
inquiry
Yield:6224-55-1 99%
Reaction Conditions:
with dihydrogen peroxide;C22H44N6Nb2O14 in methanol;water at 45; for 3 h;Catalytic behavior;Inert atmosphere;Sealed tube;
Steps:
2.2 Catalytic reactions
General procedure: General procedure. 0.5mmol of sulfide and 1.0mL of solvent (methanol or acetonitrile, concentration 0.5M) were introduced into a 3mL vial, then dinitrogen was fluxed for ca. 3 minutes. Then, while keeping the vial under a cone under N2 flux, the catalyst (1.0mol %) and the appropriate amount (2.0 or 3.0 equiv.) of 30% aqueous solution of hydrogen peroxide were added to the mixture. The sealed vial was either thermostated at 45°C through an oil bath or left at room temperature, under stirring. After 1.5 or 2.0h (in relation to the substrate), additional equivalent of oxidant was added dropwise when necessary. At the end of the reaction, the mixture was treated with 5.0mg of MnO2 to quench the excess of oxidant and, after 10 minutes, filtered. The progress of the reaction was monitored through GC-FID analysis, by withdrawing an aliquot of 20 μL and adding 5 μL of n-hexadecane (internal standard). The oxidation products were identified by comparison of their GC retention times with those of authentic samples.
References:
Bresciani, Giulio;Ciancaleoni, Gianluca;Crucianelli, Marcello;Gemmiti, Mario;Marchetti, Fabio;Pampaloni, Guido [Molecular catalysis,2021,vol. 516,art. no. 111972]
![2-Methylbenzo[b]thiophene oxide](/CAS/20180703/GIF/33945-86-7.gif)
33945-86-7
2 suppliers
inquiry

6224-55-1
5 suppliers
inquiry
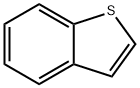
95-15-8
385 suppliers
$6.00/5g

6224-55-1
5 suppliers
inquiry
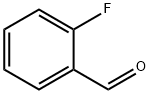
446-52-6
440 suppliers
$6.00/25g

6224-55-1
5 suppliers
inquiry